Diastereoselective Radical 1,4-Ester Migration: Radical Cyclizations of Acyclic Esters with SmI2
- PMID: 35858251
- PMCID: PMC9377304
- DOI: 10.1021/jacs.2c05972
Diastereoselective Radical 1,4-Ester Migration: Radical Cyclizations of Acyclic Esters with SmI2
Abstract
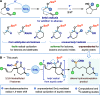
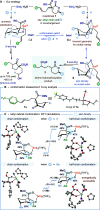
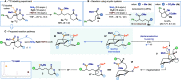
Reductive cyclizations of carbonyl compounds, mediated by samarium(II) diiodide (SmI2, Kagan's reagent), represent an invaluable platform to generate molecular complexity in a stereocontrolled manner. In addition to classical ketone and aldehyde substrates, recent advances in radical chemistry allow the cyclization of lactone and lactam-type substrates using SmI2. In contrast, acyclic esters are considered to be unreactive to SmI2 and their participation in reductive cyclizations is unprecedented. Here, we report a diastereoselective radical 1,4-ester migration process, mediated by SmI2, that delivers stereodefined alkene hydrocarboxylation products via radical cyclization of acyclic ester groups in α-carbomethoxy δ-lactones. Isotopic labeling experiments and computational studies have been used to probe the mechanism of the migration. We propose that a switch in conformation redirects single electron transfer from SmI2 to the acyclic ester group, rather than the "more reactive" lactone carbonyl. Our study paves the way for the use of elusive ketyl radicals, derived from acyclic esters, in SmI2-mediated reductive cyclizations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Sm(II)-Mediated Electron Transfer to Carboxylic Acid Derivatives: Development of Complexity-Generating Cascades.Acc Chem Res. 2015 May 19;48(5):1263-75. doi: 10.1021/acs.accounts.5b00083. Epub 2015 Apr 14. Acc Chem Res. 2015. PMID: 25871998
-
Lactone radical cyclizations and cyclization cascades mediated by SmI2-H2O.J Am Chem Soc. 2012 Aug 1;134(30):12751-7. doi: 10.1021/ja3047975. Epub 2012 Jul 18. J Am Chem Soc. 2012. PMID: 22746316
-
Studies on the mechanism, selectivity, and synthetic utility of lactone reduction using SmI(2) and H(2)O.J Am Chem Soc. 2009 Oct 28;131(42):15467-73. doi: 10.1021/ja906396u. J Am Chem Soc. 2009. PMID: 19764763
-
Synthesis of Nitrogen Heterocycles Using Samarium(II) Iodide.Molecules. 2017 Nov 21;22(11):2018. doi: 10.3390/molecules22112018. Molecules. 2017. PMID: 29160806 Free PMC article. Review.
-
SmI2-induced cyclizations and their applications in natural product synthesis.Chem Rec. 2010 Jun;10(3):159-72. doi: 10.1002/tcr.200900027. Chem Rec. 2010. PMID: 20503205 Review.
Cited by
-
Accessing Unusual Reactivity through Chelation-Promoted Bond Weakening.Inorg Chem. 2023 Mar 27;62(12):5040-5045. doi: 10.1021/acs.inorgchem.3c00298. Epub 2023 Mar 13. Inorg Chem. 2023. PMID: 36912617 Free PMC article.
-
Development of a Triethylborane Mediated Giese Cyclization/Aldol Reaction Cascade for the Total Synthesis of Ganoapplanin.Synlett. 2025 Jan 24:a-2501-4079. doi: 10.1055/a-2501-4079. Online ahead of print. Synlett. 2025. PMID: 40857544
-
Total Synthesis of Ganoapplanin Enabled by a Radical Addition/Aldol Reaction Cascade.J Am Chem Soc. 2024 Aug 21;146(33):22937-22942. doi: 10.1021/jacs.4c08291. Epub 2024 Aug 7. J Am Chem Soc. 2024. PMID: 39110664 Free PMC article.
References
-
-
For recent reviews about the topic, see:
- Plesniak M. P.; Huang H.-M.; Procter D. J. Radical cascade reactions triggered by single electron transfer. Nature Rev. Chem. 2017, 1, 0077.10.1038/s41570-017-0077. - DOI
- Miyabe H.; Kawashima A.; Yoshioka E.; Kohtani S. Progress in Enantioselective Radical Cyclizations. Chem. -Eur. J. 2017, 23, 6225.10.1002/chem.201603124. - DOI - PubMed
- Huang H.-M.; Garduño-Castro M. H.; Morrill C.; Procter D. J. Catalytic cascade reactions by radical relay. Chem. Soc. Rev. 2019, 48, 4626.10.1039/C8CS00947C. - DOI - PubMed
-
-
-
For selected reviews on SET and radical chemistry, see:
- Gansäuer A.; Bluhm H. Reagent-Controlled Transition-Metal-Catalyzed Radical Reactions. Chem. Rev. 2000, 100, 2771–2788. 10.1021/cr9902648. - DOI - PubMed
- Studer A.; Curran D. P. Catalysis of Radical Reactions: A Radical Chemistry Perspective. Angew. Chem. Int, Ed. 2016, 55, 58–102. 10.1002/anie.201505090. - DOI - PubMed
- Yan M.; Lo J. C.; Edwards J. T.; Baran P. S. Radicals: Reactive Intermediates with Translational Potential. J. Am. Chem. Soc. 2016, 138, 12692–12714. 10.1021/jacs.6b08856. - DOI - PMC - PubMed
-
-
- Girard P.; Namy J.-L.; Kagan H. B. Divalent lanthanide derivatives in organic synthesis. 1. Mild preparation of SmI2 and YbI2 and their use as reducing or coupling agents. J. Am. Chem. Soc. 1980, 102, 2693–2698. 10.1021/ja00528a029. - DOI
-
For selected reviews of SmI2 chemistry, see:
- Szostak M.; Fazakerley N. J.; Parmar D.; Procter D. J. Cross-Coupling Reactions Using Samarium(II) Iodide. Chem. Rev. 2014, 114, 5959–6039. 10.1021/cr400685r. - DOI - PubMed
- Molander G. A.; Harris C. R. Sequencing Reactions with Samarium(II) Iodide. Chem. Rev. 1996, 96, 307–338. 10.1021/cr950019y. - DOI - PubMed
- Flowers R. A. II. Mechanistic Studies on the Roles of Cosolvents and Additives in Samarium(II)-Based Reductions. Synlett 2008, 2008, 1427–1439. 10.1055/s-2008-1078414. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

