Discovery of a novel genomic alteration that renders leukemic cells resistant to CD19-targeted immunotherapies
- PMID: 35858291
- PMCID: PMC9582720
- DOI: 10.1182/bloodadvances.2022007705
Discovery of a novel genomic alteration that renders leukemic cells resistant to CD19-targeted immunotherapies
Conflict of interest statement
Figures

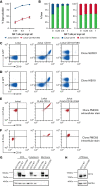
References
-
- Topp MS, Gökbuget N, Zugmaier G, et al. Phase II trial of the anti-CD19 bispecific T cell-engager blinatumomab shows hematologic and molecular remissions in patients with relapsed or refractory B-precursor acute lymphoblastic leukemia. J Clin Oncol. 2014;32(36):4134–4140. - PubMed
-
- Orlando EJ, Han X, Tribouley C, et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med. 2018;24(10):1504–1506. - PubMed

