Gd2O3-mesoporous silica/gold nanoshells: A potential dual T1/ T2 contrast agent for MRI-guided localized near-IR photothermal therapy
- PMID: 35858309
- PMCID: PMC9303993
- DOI: 10.1073/pnas.2123527119
Gd2O3-mesoporous silica/gold nanoshells: A potential dual T1/ T2 contrast agent for MRI-guided localized near-IR photothermal therapy
Abstract
A promising clinical trial utilizing gold-silica core-shell nanostructures coated with polyethylene glycol (PEG) has been reported for near-infrared (NIR) photothermal therapy (PTT) of prostate cancer. The next critical step for PTT is the visualization of therapeutically relevant nanoshell (NS) concentrations at the tumor site. Here we report the synthesis of PEGylated Gd2O3-mesoporous silica/gold core/shell NSs (Gd2O3-MS NSs) with NIR photothermal properties that also supply sufficient MRI contrast to be visualized at therapeutic doses (≥108 NSs per milliliter). The nanoparticles have r1 relaxivities more than three times larger than those of conventional T1 contrast agents, requiring less concentration of Gd3+ to observe an equivalent signal enhancement in T1-weighted MR images. Furthermore, Gd2O3-MS NS nanoparticles have r2 relaxivities comparable to those of existing T2 contrast agents, observed in agarose phantoms. This highly unusual combination of simultaneous T1 and T2 contrast allows for MRI enhancement through different approaches. As a rudimentary example, we demonstrate T1/T2 ratio MR images with sixfold contrast signal enhancement relative to its T1 MRI and induced temperature increases of 20 to 55 °C under clinical illumination conditions. These nanoparticles facilitate MRI-guided PTT while providing real-time temperature feedback through thermal MRI mapping.
Keywords: MRI contrast; gadolinium oxide; gold nanoshells; mesoporous silica; photothermal.
Conflict of interest statement
Competing interest statement: A patent is being filed on the nanoparticle whose synthesis and properties we report in this manuscript.
Figures
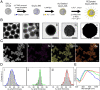
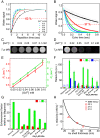
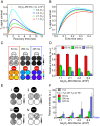


References
-
- Jain N. K., et al. , Nanoengineered photoactive theranostic agents for cancer. Nanophotonics 10, 2973–2997 (2021).
-
- Weissleder R., A clearer vision for in vivo imaging. Nat. Biotechnol. 19, 316–317 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

