Methanol biotransformation toward high-level production of fatty acid derivatives by engineering the industrial yeast Pichia pastoris
- PMID: 35858340
- PMCID: PMC9303929
- DOI: 10.1073/pnas.2201711119
Methanol biotransformation toward high-level production of fatty acid derivatives by engineering the industrial yeast Pichia pastoris
Abstract
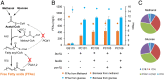
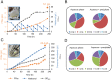
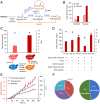
Methanol-based biorefinery is a promising strategy to achieve carbon neutrality goals by linking CO2 capture and solar energy storage. As a typical methylotroph, Pichia pastoris shows great potential in methanol biotransformation. However, challenges still remain in engineering methanol metabolism for chemical overproduction. Here, we present the global rewiring of the central metabolism for efficient production of free fatty acids (FFAs; 23.4 g/L) from methanol, with an enhanced supply of precursors and cofactors, as well as decreased accumulation of formaldehyde. Finally, metabolic transforming of the fatty acid cell factory enabled overproduction of fatty alcohols (2.0 g/L) from methanol. This study demonstrated that global metabolic rewiring released the great potential of P. pastoris for methanol biotransformation toward chemical overproduction.
Keywords: biofuels; metabolic engineering; methylotrophic yeast; oleochemicals; synthetic biotechnology.
Conflict of interest statement
Competing interest statement: P.C., and Y.J.Z. have one patent (202010516436.1) for protecting part of the work described herein. All other authors declare no competing financial interests.
Figures

Similar articles
-
Low-carbon and overproduction of cordycepin from methanol using engineered Pichia pastoris cell factory.Bioresour Technol. 2024 Dec;413:131446. doi: 10.1016/j.biortech.2024.131446. Epub 2024 Sep 5. Bioresour Technol. 2024. PMID: 39241814
-
Rewiring the methanol assimilation pathway in the methylotrophic yeast Pichia pastoris for high-level production of erythritol.Bioresour Technol. 2025 Jul;427:132430. doi: 10.1016/j.biortech.2025.132430. Epub 2025 Mar 19. Bioresour Technol. 2025. PMID: 40118222
-
Peroxisomal metabolic coupling improves fatty alcohol production from sole methanol in yeast.Proc Natl Acad Sci U S A. 2023 Mar 21;120(12):e2220816120. doi: 10.1073/pnas.2220816120. Epub 2023 Mar 13. Proc Natl Acad Sci U S A. 2023. PMID: 36913588 Free PMC article.
-
Bioconversion of C1 feedstocks for chemical production using Pichia pastoris.Trends Biotechnol. 2023 Aug;41(8):1066-1079. doi: 10.1016/j.tibtech.2023.03.006. Epub 2023 Mar 24. Trends Biotechnol. 2023. PMID: 36967258 Review.
-
Heterologous protein production in methylotrophic yeasts.Appl Microbiol Biotechnol. 2000 Dec;54(6):741-50. doi: 10.1007/s002530000464. Appl Microbiol Biotechnol. 2000. PMID: 11152064 Review.
Cited by
-
Engineering yeast for tailored fatty acid profiles.Appl Microbiol Biotechnol. 2025 Apr 22;109(1):101. doi: 10.1007/s00253-025-13487-1. Appl Microbiol Biotechnol. 2025. PMID: 40263140 Free PMC article. Review.
-
An improved CRISPRi system in Pichia pastoris.Synth Syst Biotechnol. 2023 Jul 7;8(3):479-485. doi: 10.1016/j.synbio.2023.06.008. eCollection 2023 Sep. Synth Syst Biotechnol. 2023. PMID: 37692202 Free PMC article.
-
Mechanistic investigation of a D to N mutation in DAHP synthase that dictates carbon flux into the shikimate pathway in yeast.Commun Chem. 2023 Jul 15;6(1):152. doi: 10.1038/s42004-023-00946-x. Commun Chem. 2023. PMID: 37454208 Free PMC article.
-
Biosynthesis of High-Active Hemoproteins by the Efficient Heme-Supply Pichia Pastoris Chassis.Adv Sci (Weinh). 2023 Oct;10(30):e2302826. doi: 10.1002/advs.202302826. Epub 2023 Aug 30. Adv Sci (Weinh). 2023. PMID: 37649147 Free PMC article.
-
CRISPR/Cas9-based toolkit for rapid marker recycling and combinatorial libraries in Komagataella phaffii.Appl Microbiol Biotechnol. 2024 Feb 7;108(1):197. doi: 10.1007/s00253-024-13037-1. Appl Microbiol Biotechnol. 2024. PMID: 38324086 Free PMC article.
References
-
- Zhou Y. J., Kerkhoven E. J., Nielsen J., Barriers and opportunities in bio-based production of hydrocarbons. Nat. Energy 3, 925–935 (2018).
-
- Lais A., Gondal M. A., Dastageer M. A., Al-Adel F. F., Experimental parameters affecting the photocatalytic reduction performance of CO2 to methanol: A review. Int. J. Energy Res. 42, 2031–2049 (2018).
-
- Al-Rowaili F. N., Jamal A., Ba Shammakh M. S., Rana A., A review on recent advances for electrochemical reduction of carbon dioxide to methanol using metal–organic framework (MOF) and non-MOF catalysts: Challenges and future prospects. ACS Sustain. Chem. Eng. 6, 15895–15914 (2018).
-
- Whitaker W. B., Sandoval N. R., Bennett R. K., Fast A. G., Papoutsakis E. T., Synthetic methylotrophy: Engineering the production of biofuels and chemicals based on the biology of aerobic methanol utilization. Curr. Opin. Biotechnol. 33, 165–175 (2015). - PubMed
-
- Wang Y., Fan L., Tuyishime P., Zheng P., Sun J., Synthetic methylotrophy: A practical solution for methanol-based biomanufacturing. Trends Biotechnol. 38, 650–666 (2020). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

