Tropism of SARS-CoV-2 for human cortical astrocytes
- PMID: 35858406
- PMCID: PMC9335272
- DOI: 10.1073/pnas.2122236119
Tropism of SARS-CoV-2 for human cortical astrocytes
Abstract
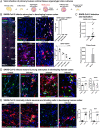
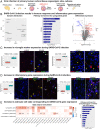
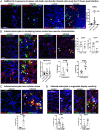
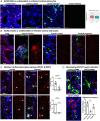
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) readily infects a variety of cell types impacting the function of vital organ systems, with particularly severe impact on respiratory function. Neurological symptoms, which range in severity, accompany as many as one-third of COVID-19 cases, indicating a potential vulnerability of neural cell types. To assess whether human cortical cells can be directly infected by SARS-CoV-2, we utilized stem-cell-derived cortical organoids as well as primary human cortical tissue, both from developmental and adult stages. We find significant and predominant infection in cortical astrocytes in both primary tissue and organoid cultures, with minimal infection of other cortical populations. Infected and bystander astrocytes have a corresponding increase in inflammatory gene expression, reactivity characteristics, increased cytokine and growth factor signaling, and cellular stress. Although human cortical cells, particularly astrocytes, have no observable ACE2 expression, we find high levels of coronavirus coreceptors in infected astrocytes, including CD147 and DPP4. Decreasing coreceptor abundance and activity reduces overall infection rate, and increasing expression is sufficient to promote infection. Thus, we find tropism of SARS-CoV-2 for human astrocytes resulting in inflammatory gliosis-type injury that is dependent on coronavirus coreceptors.
Keywords: SARS-CoV-2 tropism; astrocyte reactivity; organoid models.
Conflict of interest statement
Competing interest statement: A.R.K. is a co-founder, consultant, and member of the Board of Neurona Therapeutics. The other authors declare no competing interest.
Figures

Update of
-
Tropism of SARS-CoV-2 for Developing Human Cortical Astrocytes.bioRxiv [Preprint]. 2021 Jan 18:2021.01.17.427024. doi: 10.1101/2021.01.17.427024. bioRxiv. 2021. Update in: Proc Natl Acad Sci U S A. 2022 Jul 26;119(30):e2122236119. doi: 10.1073/pnas.2122236119. PMID: 33469577 Free PMC article. Updated. Preprint.
Comment in
-
In SARS-CoV-2, astrocytes are in it for the long haul.Proc Natl Acad Sci U S A. 2022 Jul 26;119(30):e2209130119. doi: 10.1073/pnas.2209130119. Epub 2022 Jul 18. Proc Natl Acad Sci U S A. 2022. PMID: 35858460 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

