Competition between growth and shear stress drives intermittency in preferential flow paths in porous medium biofilms
- PMID: 35858419
- PMCID: PMC9335220
- DOI: 10.1073/pnas.2122202119
Competition between growth and shear stress drives intermittency in preferential flow paths in porous medium biofilms
Abstract
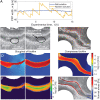
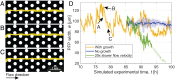
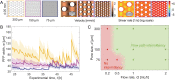
Bacteria in porous media, such as soils, aquifers, and filters, often form surface-attached communities known as biofilms. Biofilms are affected by fluid flow through the porous medium, for example, for nutrient supply, and they, in turn, affect the flow. A striking example of this interplay is the strong intermittency in flow that can occur when biofilms nearly clog the porous medium. Intermittency manifests itself as the rapid opening and slow closing of individual preferential flow paths (PFPs) through the biofilm-porous medium structure, leading to continual spatiotemporal rearrangement. The drastic changes to the flow and mass transport induced by intermittency can affect the functioning and efficiency of natural and industrial systems. Yet, the mechanistic origin of intermittency remains unexplained. Here, we show that the mechanism driving PFP intermittency is the competition between microbial growth and shear stress. We combined microfluidic experiments quantifying Bacillus subtilis biofilm formation and behavior in synthetic porous media for different pore sizes and flow rates with a mathematical model accounting for flow through the biofilm and biofilm poroelasticity to reveal the underlying mechanisms. We show that the closing of PFPs is driven by microbial growth, controlled by nutrient mass flow. Opposing this, we find that the opening of PFPs is driven by flow-induced shear stress, which increases as a PFP becomes narrower due to microbial growth, causing biofilm compression and rupture. Our results demonstrate that microbial growth and its competition with shear stresses can lead to strong temporal variability in flow and transport conditions in bioclogged porous media.
Keywords: bacterial biofilms; bioclogging; biofilm dynamics; biofouling; porous medium flow.
Conflict of interest statement
The authors declare no competing interest.
Figures


Similar articles
-
Morphogenesis of Biofilms in Porous Media and Control on Hydrodynamics.Environ Sci Technol. 2023 Apr 11;57(14):5666-5677. doi: 10.1021/acs.est.2c08890. Epub 2023 Mar 28. Environ Sci Technol. 2023. PMID: 36976631
-
A Microfluidic Platform to Study Bioclogging in Porous Media.J Vis Exp. 2022 Oct 13;(188). doi: 10.3791/64689. J Vis Exp. 2022. PMID: 36314844
-
Biofilms in 3D porous media: Delineating the influence of the pore network geometry, flow and mass transfer on biofilm development.Water Res. 2018 May 1;134:280-291. doi: 10.1016/j.watres.2018.01.059. Epub 2018 Feb 5. Water Res. 2018. PMID: 29433078
-
Biofilm development and the dynamics of preferential flow paths in porous media.Biofouling. 2013;29(9):1069-86. doi: 10.1080/08927014.2013.828284. Epub 2013 Sep 13. Biofouling. 2013. PMID: 24028574
-
Applying the handicap principle to biofilms: condition-dependent signalling in Bacillus subtilis microbial communities.Environ Microbiol. 2019 Feb;21(2):531-540. doi: 10.1111/1462-2920.14497. Epub 2019 Jan 8. Environ Microbiol. 2019. PMID: 30536518 Review.
Cited by
-
Rapid formation of bioaggregates and morphology transition to biofilm streamers induced by pore-throat flows.Proc Natl Acad Sci U S A. 2023 Apr 4;120(14):e2204466120. doi: 10.1073/pnas.2204466120. Epub 2023 Mar 29. Proc Natl Acad Sci U S A. 2023. PMID: 36989304 Free PMC article.
-
Microfluidic investigation of the impacts of flow fluctuations on the development of Pseudomonas putida biofilms.NPJ Biofilms Microbiomes. 2023 Oct 3;9(1):73. doi: 10.1038/s41522-023-00442-z. NPJ Biofilms Microbiomes. 2023. PMID: 37789000 Free PMC article.
-
The mechanism of biofilm detachment in porous medium under flow field.Biomicrofluidics. 2024 May 6;18(3):034103. doi: 10.1063/5.0203061. eCollection 2024 May. Biomicrofluidics. 2024. PMID: 38737754 Free PMC article.
-
Efficiency, Microbial Communities, and Nitrogen Metabolism in Denitrification Biological Filter: Insights into Varied Pore Ceramsite Media.Microorganisms. 2025 May 23;13(6):1187. doi: 10.3390/microorganisms13061187. Microorganisms. 2025. PMID: 40572075 Free PMC article.
-
The mitigation of spatial constraint in porous environments enhances biofilm phylogenetic and functional diversity.Microbiome. 2025 Mar 24;13(1):84. doi: 10.1186/s40168-025-02075-0. Microbiome. 2025. PMID: 40128897 Free PMC article.
References
-
- Cunningham A. B., Sharp R. R., Hiebert R., James G., Subsurface biofilm barriers for the containment and remediation of contaminated groundwater. Bioremediat. J. 7, 151–164 (2003).
-
- Stewart T. L., Scott Fogler H., Pore-scale investigation of biomass plug development and propagation in porous media. Biotechnol. Bioeng. 77, 577–588 (2002). - PubMed
-
- Baveye P., Vandevivere P., Hoyle B. L., DeLeo P. C., Sanchez De Lozada D., Environmental impact and mechanisms of the biological clogging of saturated soils and aquifer materials. Crit. Rev. Environ. Sci. Technol. 28, 123–191 (1998).
-
- Mitchell J. K., Santamarina J. C., Biological considerations in geotechnical engineering. J. Geotech. Geoenviron. Eng. 131, 1222–1233 (2005).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

