Chirality enhances oxygen reduction
- PMID: 35858429
- PMCID: PMC9335305
- DOI: 10.1073/pnas.2202650119
Chirality enhances oxygen reduction
Abstract
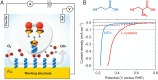
Controlled reduction of oxygen is important for developing clean energy technologies, such as fuel cells, and is vital to the existence of aerobic organisms. The process starts with oxygen in a triplet ground state and ends with products that are all in singlet states. Hence, spin constraints in the oxygen reduction must be considered. Here, we show that the electron transfer efficiency from chiral electrodes to oxygen (oxygen reduction reaction) is enhanced over that from achiral electrodes. We demonstrate lower overpotentials and higher current densities for chiral catalysts versus achiral ones. This finding holds even for electrodes composed of heavy metals with large spin-orbit coupling. The effect results from the spin selectivity conferred on the electron current by the chiral assemblies, the chiral-induced spin selectivity effect.
Keywords: chirality; fuel cells; oxygen; respiration; spin.
Conflict of interest statement
The authors declare no competing interest.
Figures



Similar articles
-
Chiral Induced Spin Selectivity and Its Implications for Biological Functions.Annu Rev Biophys. 2022 May 9;51:99-114. doi: 10.1146/annurev-biophys-083021-070400. Epub 2021 Dec 21. Annu Rev Biophys. 2022. PMID: 34932912 Review.
-
Direct control of electron spin at an intrinsically chiral surface for highly efficient oxygen reduction reaction.Proc Natl Acad Sci U S A. 2025 Mar 4;122(9):e2413609122. doi: 10.1073/pnas.2413609122. Epub 2025 Feb 25. Proc Natl Acad Sci U S A. 2025. PMID: 39999173 Free PMC article.
-
Chirality Assisted Triplet Electron Pairing.J Phys Chem Lett. 2025 Feb 13;16(6):1629-1633. doi: 10.1021/acs.jpclett.4c03734. Epub 2025 Feb 5. J Phys Chem Lett. 2025. PMID: 39907703 Free PMC article.
-
Spin-Dependent Transport through Chiral Molecules Studied by Spin-Dependent Electrochemistry.Acc Chem Res. 2016 Nov 15;49(11):2560-2568. doi: 10.1021/acs.accounts.6b00446. Epub 2016 Oct 24. Acc Chem Res. 2016. PMID: 27797176 Free PMC article.
-
[Spin-catalysis in the processes of photo- and bioactivation of molecular oxygen].Ukr Biokhim Zh (1999). 2009 May-Jun;81(3):21-45. Ukr Biokhim Zh (1999). 2009. PMID: 19877428 Review. Russian.
Cited by
-
Chiral "doped" MOFs: an electrochemical and theoretical integrated study.Front Chem. 2023 Aug 8;11:1215619. doi: 10.3389/fchem.2023.1215619. eCollection 2023. Front Chem. 2023. PMID: 37614707 Free PMC article.
-
Does Coherence Affect the Multielectron Oxygen Reduction Reaction?J Phys Chem Lett. 2023 Oct 26;14(42):9377-9384. doi: 10.1021/acs.jpclett.3c02594. Epub 2023 Oct 12. J Phys Chem Lett. 2023. PMID: 37824289 Free PMC article.
-
The chirality-induced spin selectivity effect in asymmetric spin transport: from solution to device applications.Chem Sci. 2024 Oct 29;15(45):18751-18771. doi: 10.1039/d4sc05736h. eCollection 2024 Nov 20. Chem Sci. 2024. PMID: 39568626 Free PMC article. Review.
-
Elucidating the Chirality-Induced Spin Selectivity Effect of Co-Doped NiO Deposited on Ni Foam for Highly Stable Zn-Air Batteries.ACS Appl Mater Interfaces. 2025 Mar 26;17(12):18228-18242. doi: 10.1021/acsami.4c20630. Epub 2025 Mar 13. ACS Appl Mater Interfaces. 2025. PMID: 40080125 Free PMC article.
-
Chiral Induced Spin Selectivity.Chem Rev. 2024 Feb 28;124(4):1950-1991. doi: 10.1021/acs.chemrev.3c00661. Epub 2024 Feb 16. Chem Rev. 2024. PMID: 38364021 Free PMC article. Review.
References
-
- Kulkarni A., Siahrostami S., Patel A., Nørskov J. K., Understanding catalytic activity trends in the oxygen reduction reaction. Chem. Rev. 118, 2302–2312 (2018). - PubMed
-
- Shao M., Chang Q., Dodelet J. P., Chenitz R., Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 116, 3594–3657 (2016). - PubMed
-
- Dai L., Xue Y., Qu L., Choi H. J., Baek J. B., Metal-free catalysts for oxygen reduction reaction. Chem. Rev. 115, 4823–4892 (2015). - PubMed
-
- Stamenkovic V. R., Strmcnik D., Lopes P. P., Markovic N. M., Energy and fuels from electrochemical interfaces. Nat. Mater. 16, 57–69 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

