Pain hypersensitivity in a pharmacological mouse model of attention-deficit/hyperactivity disorder
- PMID: 35858441
- PMCID: PMC9335339
- DOI: 10.1073/pnas.2114094119
Pain hypersensitivity in a pharmacological mouse model of attention-deficit/hyperactivity disorder
Abstract
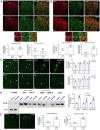
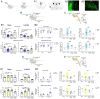
Clinical evidence suggests that pain hypersensitivity develops in patients with attention-deficit/hyperactivity disorder (ADHD). However, the mechanisms and neural circuits involved in these interactions remain unknown because of the paucity of studies in animal models. We previously validated a mouse model of ADHD obtained by neonatal 6-hydroxydopamine (6-OHDA) injection. Here, we have demonstrated that 6-OHDA mice exhibit a marked sensitization to thermal and mechanical stimuli, suggesting that phenotypes associated with ADHD include increased nociception. Moreover, sensitization to pathological inflammatory stimulus is amplified in 6-OHDA mice as compared to shams. In this ADHD model, spinal dorsal horn neuron hyperexcitability was observed. Furthermore, ADHD-related hyperactivity and anxiety, but not inattention and impulsivity, are worsened in persistent inflammatory conditions. By combining in vivo electrophysiology, optogenetics, and behavioral analyses, we demonstrated that anterior cingulate cortex (ACC) hyperactivity alters the ACC-posterior insula circuit and triggers changes in spinal networks that underlie nociceptive sensitization. Altogether, our results point to shared mechanisms underlying the comorbidity between ADHD and nociceptive sensitization. This interaction reinforces nociceptive sensitization and hyperactivity, suggesting that overlapping ACC circuits may be targeted to develop better treatments.
Keywords: anterior cingulate cortex; attention-deficit/hyperactivity disorder; comorbidity; pain sensitization; spinal cord.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Biederman J., Attention-deficit/hyperactivity disorder: A selective overview. Biol. Psychiatry 57, 1215–1220 (2005). - PubMed
-
- American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, Washington, DC, ed. 5, 2013).
-
- Kooij J. J., et al. , Distinguishing comorbidity and successful management of adult ADHD. J. Atten. Disord. 16, 3S–19S (2012). - PubMed
-
- Schatz D. B., Rostain A. L., ADHD with comorbid anxiety: A review of the current literature. J. Atten. Disord. 10, 141–149 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

