Immune correlates of HIV-1 reservoir cell decline in early-treated infants
- PMID: 35858580
- PMCID: PMC9314543
- DOI: 10.1016/j.celrep.2022.111126
Immune correlates of HIV-1 reservoir cell decline in early-treated infants
Abstract
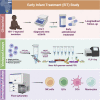
Initiation of antiretroviral therapy (ART) in infected neonates within hours after birth limits viral reservoir seeding but does not prevent long-term HIV-1 persistence. Here, we report parallel assessments of HIV-1 reservoir cells and innate antiviral immune responses in a unique cohort of 37 infected neonates from Botswana who started ART extremely early, frequently within hours after birth. Decline of genome-intact HIV-1 proviruses occurs rapidly after initiation of ART and is associated with an increase in natural killer (NK) cell populations expressing the cytotoxicity marker CD57 and with a decrease in NK cell subsets expressing the inhibitory marker NKG2A. Immune perturbations in innate lymphoid cells, myeloid dendritic cells, and monocytes detected at birth normalize after rapid institution of antiretroviral therapy but do not notably influence HIV-1 reservoir cell dynamics. These results suggest that HIV-1 reservoir cell seeding and evolution in early-treated neonates is markedly influenced by antiviral NK cell immune responses.
Keywords: CP: Immunology; CP: Microbiology; HIV reservoir; NK cells; innate immune responses; intact HIV-1 proviruses; pediatric HIV-1 infection.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests D.R.K. has received consulting honoraria and/or research support from Gilead, Merck, and ViiV. M.L. has received speaking and consulting honoraria from Merck.
Figures




References
-
- Ananworanich J., Puthanakit T., Suntarattiwong P., Chokephaibulkit K., Kerr S.J., Fromentin R., Bakeman W., Intasan J., Mahanontharit A., Sirivichayakul S., et al. Reduced markers of HIV persistence and restricted HIV-specific immune responses after early antiretroviral therapy in children. AIDS. 2014;28:1015–1020. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI078799/AI/NIAID NIH HHS/United States
- K24 AI155233/AI/NIAID NIH HHS/United States
- U01 AI135940/AI/NIAID NIH HHS/United States
- R01 HL134539/HL/NHLBI NIH HHS/United States
- U01 AI114235/AI/NIAID NIH HHS/United States
- R01 AI098487/AI/NIAID NIH HHS/United States
- R61 DA047034/DA/NIDA NIH HHS/United States
- R33 AI116228/AI/NIAID NIH HHS/United States
- HHSN261200800001E/CA/NCI NIH HHS/United States
- R21 AI120850/AI/NIAID NIH HHS/United States
- R33 DA047034/DA/NIDA NIH HHS/United States
- R01 AI152979/AI/NIAID NIH HHS/United States
- U01 AI117841/AI/NIAID NIH HHS/United States
- R01 DK120387/DK/NIDDK NIH HHS/United States
- R56 AI125109/AI/NIAID NIH HHS/United States
- R01 AI120008/AI/NIAID NIH HHS/United States
- HHSN261200800001C/RC/CCR NIH HHS/United States
- R21 AI116228/AI/NIAID NIH HHS/United States
- UM1 AI164566/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

