Vagus nerve stimulation drives selective circuit modulation through cholinergic reinforcement
- PMID: 35858623
- PMCID: PMC10212211
- DOI: 10.1016/j.neuron.2022.06.017
Vagus nerve stimulation drives selective circuit modulation through cholinergic reinforcement
Abstract
Vagus nerve stimulation (VNS) is a neuromodulation therapy for a broad and expanding set of neurologic conditions. However, the mechanism through which VNS influences central nervous system circuitry is not well described, limiting therapeutic optimization. VNS leads to widespread brain activation, but the effects on behavior are remarkably specific, indicating plasticity unique to behaviorally engaged neural circuits. To understand how VNS can lead to specific circuit modulation, we leveraged genetic tools including optogenetics and in vivo calcium imaging in mice learning a skilled reach task. We find that VNS enhances skilled motor learning in healthy animals via a cholinergic reinforcement mechanism, producing a rapid consolidation of an expert reach trajectory. In primary motor cortex (M1), VNS drives precise temporal modulation of neurons that respond to behavioral outcome. This suggests that VNS may accelerate motor refinement in M1 via cholinergic signaling, opening new avenues for optimizing VNS to target specific disease-relevant circuitry.
Keywords: basal forebrain; cholinergic; learning; motor cortex; motor learning; neuromodulation; outcome; plasticity; reinforcement; vagus nerve stimulation.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



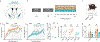

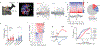


Comment in
-
Why study mechanisms of brain stimulation therapies? To modulate the right neurons, in the right way, at the right time.Neuron. 2022 Sep 7;110(17):2709-2712. doi: 10.1016/j.neuron.2022.08.004. Neuron. 2022. PMID: 36076336
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

