Plasmacytoid dendritic cells during COVID-19: Ally or adversary?
- PMID: 35858624
- PMCID: PMC9279298
- DOI: 10.1016/j.celrep.2022.111148
Plasmacytoid dendritic cells during COVID-19: Ally or adversary?
Abstract
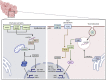
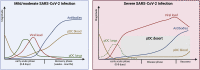
Plasmacytoid dendritic cells (pDCs) are specialized cells of the immune system that are thought to be the main cellular source of type I interferon alpha (IFNα) in response to viral infections. IFNs are powerful antivirals, whereas defects in their function or induction lead to impaired resistance to virus infections, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19. IFN production needs to be controlled, because sustained IFN production can also have detrimental effects on disease outcome. As such, pDCs are likely important for acute antiviral protection against SARS-CoV-2 infection but could potentially also contribute to chronic IFN levels. Here, we provide a historical overview of pDC biology and summarize existing literature addressing their involvement and importance during viral infections of the airways. Furthermore, we outline recent reports focused on the potential role of pDCs during SARS-CoV-2 infection, as well as the potential for this cellular subset to impact COVID-19 disease outcome.
Keywords: COVID-19; CP: Immunology; CP: Microbiology; SARS-CoV-2; antiviral responses; inflammation; plasmacytoid dendritic cells.
Copyright © 2022 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests M.R.J. is a founder and shareholder of UNIKUM Therapeutics. The remaining authors declare no competing interests.
Figures
Similar articles
-
Severe COVID-19 patients have impaired plasmacytoid dendritic cell-mediated control of SARS-CoV-2.Nat Commun. 2023 Feb 8;14(1):694. doi: 10.1038/s41467-023-36140-9. Nat Commun. 2023. PMID: 36755036 Free PMC article.
-
Sensing of SARS-CoV-2-infected cells by plasmacytoid dendritic cells is modulated via an interplay between CD54/ICAM-1 and CD11a/LFA-1 αL integrin.J Virol. 2025 Feb 25;99(2):e0123524. doi: 10.1128/jvi.01235-24. Epub 2025 Jan 13. J Virol. 2025. PMID: 39804090 Free PMC article.
-
Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2.mBio. 2020 Sep 10;11(5):e01928-20. doi: 10.1128/mBio.01928-20. mBio. 2020. PMID: 32913009 Free PMC article.
-
Interplay between SARS-CoV-2 and the type I interferon response.PLoS Pathog. 2020 Jul 29;16(7):e1008737. doi: 10.1371/journal.ppat.1008737. eCollection 2020 Jul. PLoS Pathog. 2020. PMID: 32726355 Free PMC article. Review.
-
Correlation between Type I Interferon Associated Factors and COVID-19 Severity.Int J Mol Sci. 2022 Sep 19;23(18):10968. doi: 10.3390/ijms231810968. Int J Mol Sci. 2022. PMID: 36142877 Free PMC article. Review.
Cited by
-
Single-Cell Gene Expression Analysis Revealed Immune Cell Signatures of Delta COVID-19.Cells. 2022 Sep 21;11(19):2950. doi: 10.3390/cells11192950. Cells. 2022. PMID: 36230912 Free PMC article.
-
Microplastics dysregulate innate immunity in the SARS-CoV-2 infected lung.Front Immunol. 2024 May 13;15:1382655. doi: 10.3389/fimmu.2024.1382655. eCollection 2024. Front Immunol. 2024. PMID: 38803494 Free PMC article.
-
T-cell immunity to SARS-CoV-2: what if the known best is not the optimal course for the long run? Adapting to evolving targets.Front Immunol. 2023 Jun 14;14:1133225. doi: 10.3389/fimmu.2023.1133225. eCollection 2023. Front Immunol. 2023. PMID: 37388738 Free PMC article. Review.
-
Suppression of Type I Interferon Signaling in Myeloid Cells by Autoantibodies in Severe COVID-19 Patients.J Clin Immunol. 2024 Apr 22;44(4):104. doi: 10.1007/s10875-024-01708-7. J Clin Immunol. 2024. PMID: 38647550 Free PMC article.
-
ICOS-ICOSL pathway enhances NKT-like cell antiviral function in pregnant women with COVID-19.Int J Med Sci. 2024 Jul 16;21(10):1890-1902. doi: 10.7150/ijms.95952. eCollection 2024. Int J Med Sci. 2024. PMID: 39113896 Free PMC article.
References
-
- Ader F., Peiffer-Smadja N., Poissy J., Bouscambert-Duchamp M., Belhadi D., Diallo A., Delmas C., Saillard J., Dechanet A., Mercier N., Dupont A., et al. An open-label randomized controlled trial of the effect of lopinavir/ritonavir, lopinavir/ritonavir plus IFN-beta-1a and hydroxychloroquine in hospitalized patients with COVID-19. Clin. Microbiol. Infect. 2021;27:1826–1837. - PMC - PubMed
-
- Alculumbre S.G., Saint-André V., Di Domizio J., Vargas P., Sirven P., Bost P., Maurin M., Maiuri P., Wery M., Roman M.S., et al. Diversification of human plasmacytoid predendritic cells in response to a single stimulus. Nat. Immunol. 2018;19:63–75. - PubMed
-
- Anjum F.R., Anam S., Abbas G., Mahmood M.S., Rahman S.U., Goraya M.U., Abdullah R.M., Luqman M., Ali A., Akram M.K., Chaudhry T.H. Type I IFNs: a blessing in disguise or partner in crime in MERS-CoV-SARS-CoV-and SARS-CoV-2-induced pathology and potential use of type I IFNs in synergism with IFN-gamma as a novel antiviral approach against COVID-19. Viral Immunol. 2021;34:321–329. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

