Comparative effectiveness of ChAdOx1 versus BNT162b2 covid-19 vaccines in health and social care workers in England: cohort study using OpenSAFELY
- PMID: 35858680
- PMCID: PMC9295078
- DOI: 10.1136/bmj-2021-068946
Comparative effectiveness of ChAdOx1 versus BNT162b2 covid-19 vaccines in health and social care workers in England: cohort study using OpenSAFELY
Abstract
Objective: To compare the effectiveness of the BNT162b2 mRNA (Pfizer-BioNTech) and the ChAdOx1 (Oxford-AstraZeneca) covid-19 vaccines against infection and covid-19 disease in health and social care workers.
Design: Cohort study, emulating a comparative effectiveness trial, on behalf of NHS England.
Setting: Linked primary care, hospital, and covid-19 surveillance records available within the OpenSAFELY-TPP research platform, covering a period when the SARS-CoV-2 Alpha variant was dominant.
Participants: 317 341 health and social care workers vaccinated between 4 January and 28 February 2021, registered with a general practice using the TPP SystmOne clinical information system in England, and not clinically extremely vulnerable.
Interventions: Vaccination with either BNT162b2 or ChAdOx1 administered as part of the national covid-19 vaccine roll-out.
Main outcome measures: Recorded SARS-CoV-2 positive test, or covid-19 related attendance at an accident and emergency (A&E) department or hospital admission occurring within 20 weeks of receipt of the first vaccine dose.
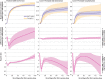
Results: Over the duration of 118 771 person-years of follow-up there were 6962 positive SARS-CoV-2 tests, 282 covid-19 related A&E attendances, and 166 covid-19 related hospital admissions. The cumulative incidence of each outcome was similar for both vaccines during the first 20 weeks after vaccination. The cumulative incidence of recorded SARS-CoV-2 infection 20 weeks after first-dose vaccination with BNT162b2 was 21.7 per 1000 people (95% confidence interval 20.9 to 22.4) and with ChAdOx1 was 23.7 (21.8 to 25.6), representing a difference of 2.04 per 1000 people (0.04 to 4.04). The difference in the cumulative incidence per 1000 people of covid-19 related A&E attendance at 20 weeks was 0.06 per 1000 people (95% CI -0.31 to 0.43). For covid-19 related hospital admission, this difference was 0.11 per 1000 people (-0.22 to 0.44).
Conclusions: In this cohort of healthcare workers where we would not anticipate vaccine type to be related to health status, we found no substantial differences in the incidence of SARS-CoV-2 infection or covid-19 disease up to 20 weeks after vaccination. Incidence dropped sharply at 3-4 weeks after vaccination, and there were few covid-19 related hospital attendance and admission events after this period. This is in line with expected onset of vaccine induced immunity and suggests strong protection against Alpha variant covid-19 disease for both vaccines in this relatively young and healthy population of healthcare workers.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form and declare the following: BG has received research funding from Health Data Research UK (HDRUK), the Laura and John Arnold Foundation, the Wellcome Trust, the NIHR Oxford Biomedical Research Centre, the NHS National Institute for Health Research School of Primary Care Research, the Mohn-Westlake Foundation, the Good Thinking Foundation, the Health Foundation, and the World Health Organisation; he also receives personal income from speaking and writing for lay audiences on the misuse of science. IJD holds shares in GlaxoSmithKline (GSK).
Figures

References
-
- Voysey M, Clemens SAC, Madhi SA, et al. Oxford COVID Vaccine Trial Group . Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99-111. 10.1016/S0140-6736(20)32661-1 - DOI - PMC - PubMed
-
- Curtis HJ, Inglesby P, Morton CE, et al. Trends and clinical characteristics of COVID-19 vaccine recipients: a federated analysis of 57.9 million patients’ primary care records in situ using OpenSAFELY. 2021. https://www.medrxiv.org/content/10.1101/2021.01.25.21250356v3. - DOI - PMC - PubMed
-
- UK Health Security Agency. COVID-19: the green book, chapter 14a. 2020. https://www.gov.uk/government/publications/covid-19-the-green-book-chapt....
-
- World Health Organization. Emergency use ICD codes for COVID-19 disease outbreak. 2020. https://www.who.int/standards/classifications/classification-of-diseases....
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
