Waning effectiveness of BNT162b2 and ChAdOx1 covid-19 vaccines over six months since second dose: OpenSAFELY cohort study using linked electronic health records
- PMID: 35858698
- PMCID: PMC10441183
- DOI: 10.1136/bmj-2022-071249
Waning effectiveness of BNT162b2 and ChAdOx1 covid-19 vaccines over six months since second dose: OpenSAFELY cohort study using linked electronic health records
Abstract
Objective: To estimate waning of covid-19 vaccine effectiveness over six months after second dose.
Design: Cohort study, approved by NHS England.
Setting: Linked primary care, hospital, and covid-19 records within the OpenSAFELY-TPP database.
Participants: Adults without previous SARS-CoV-2 infection were eligible, excluding care home residents and healthcare professionals.
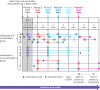
Exposures: People who had received two doses of BNT162b2 or ChAdOx1 (administered during the national vaccine rollout) were compared with unvaccinated people during six consecutive comparison periods, each of four weeks.
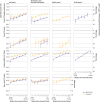
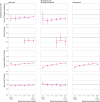
Main outcome measures: Adjusted hazard ratios for covid-19 related hospital admission, covid-19 related death, positive SARS-CoV-2 test, and non-covid-19 related death comparing vaccinated with unvaccinated people. Waning vaccine effectiveness was quantified as ratios of adjusted hazard ratios per four week period, separately for subgroups aged ≥65 years, 18-64 years and clinically vulnerable, 40-64 years, and 18-39 years.
Results: 1 951 866 and 3 219 349 eligible adults received two doses of BNT162b2 and ChAdOx1, respectively, and 2 422 980 remained unvaccinated. Waning of vaccine effectiveness was estimated to be similar across outcomes and vaccine brands. In the ≥65 years subgroup, ratios of adjusted hazard ratios for covid-19 related hospital admission, covid-19 related death, and positive SARS-CoV-2 test ranged from 1.19 (95% confidence interval 1.14 to 1.24)to 1.34 (1.09 to 1.64) per four weeks. Despite waning vaccine effectiveness, rates of covid-19 related hospital admission and death were substantially lower among vaccinated than unvaccinated adults up to 26 weeks after the second dose, with estimated vaccine effectiveness ≥80% for BNT162b2, and ≥75% for ChAdOx1. By weeks 23-26, rates of positive SARS-CoV-2 test in vaccinated people were similar to or higher than in unvaccinated people (adjusted hazard ratios up to 1.72 (1.11 to 2.68) for BNT162b2 and 1.86 (1.79 to 1.93) for ChAdOx1).
Conclusions: The rate at which estimated vaccine effectiveness waned was consistent for covid-19 related hospital admission, covid-19 related death, and positive SARS-CoV-2 test and was similar across subgroups defined by age and clinical vulnerability. If sustained to outcomes of infection with the omicron variant and to booster vaccination, these findings will facilitate scheduling of booster vaccination.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: funding for this work from the Longitudinal Health and Wellbeing COVID-19 National Core Study, Asthma UK, and the NIHR; BG has received research funding from the Laura and John Arnold Foundation, the NIHR, the NIHR School of Primary Care Research, the NIHR Oxford Biomedical Research Centre, the Mohn-Westlake Foundation, NIHR Applied Research Collaboration Oxford and Thames Valley, the Wellcome Trust, the Good Thinking Foundation, Health Data Research UK, the Health Foundation, the World Health Organization, UKRI, Asthma UK, the British Lung Foundation, and the Longitudinal Health and Wellbeing strand of the National Core Studies programme; he receives personal income from speaking and writing for lay audiences on the misuse of science; he is also a non-executive director of NHS Digital; AM is on the NHS Digital Professional Advisory Group (representing the Royal College of General Practitioners), advising on the use of general practice data for covid-19 related research and planning; until September 2019 he was interim chief medical officer of NHS Digital.
Figures
References
-
- Voysey M, Clemens SAC, Madhi SA, et al. Oxford COVID Vaccine Trial Group . Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397:99-111. 10.1016/S0140-6736(20)32661-1 - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
