Community seroprevalence of SARS-CoV-2 in children and adolescents in England, 2019-2021
- PMID: 35858775
- PMCID: PMC9887370
- DOI: 10.1136/archdischild-2022-324375
Community seroprevalence of SARS-CoV-2 in children and adolescents in England, 2019-2021
Abstract
Objective: To understand community seroprevalence of SARS-CoV-2 in children and adolescents. This is vital to understanding the susceptibility of this cohort to COVID-19 and to inform public health policy for disease control such as immunisation.
Design: We conducted a community-based cross-sectional seroprevalence study in participants aged 0-18 years old recruiting from seven regions in England between October 2019 and June 2021 and collecting extensive demographic and symptom data. Serum samples were tested for antibodies against SARS-CoV-2 spike and nucleocapsid proteins using Roche assays processed at UK Health Security Agency laboratories. Prevalence estimates were calculated for six time periods and were standardised by age group, ethnicity and National Health Service region.
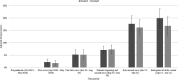
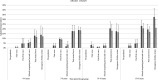
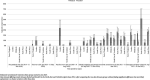
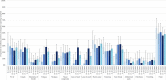
Results: Post-first wave (June-August 2020), the (anti-spike IgG) adjusted seroprevalence was 5.2%, varying from 0.9% (participants 10-14 years old) to 9.5% (participants 5-9 years old). By April-June 2021, this had increased to 19.9%, varying from 13.9% (participants 0-4 years old) to 32.7% (participants 15-18 years old). Minority ethnic groups had higher risk of SARS-CoV-2 seropositivity than white participants (OR 1.4, 95% CI 1.0 to 2.0), after adjusting for sex, age, region, time period, deprivation and urban/rural geography. In children <10 years, there were no symptoms or symptom clusters that reliably predicted seropositivity. Overall, 48% of seropositive participants with complete questionnaire data recalled no symptoms between February 2020 and their study visit.
Conclusions: Approximately one-third of participants aged 15-18 years old had evidence of antibodies against SARS-CoV-2 prior to the introduction of widespread vaccination. These data demonstrate that ethnic background is independently associated with risk of SARS-CoV-2 infection in children.
Trial registration number: NCT04061382.
Keywords: COVID-19; epidemiology; healthcare disparities; paediatrics.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: MDS acts on behalf of the University of Oxford as an investigator on studies funded or sponsored by vaccine manufacturers, including AstraZeneca, GlaxoSmithKline, Pfizer, Novavax, Janssen, Medimmune and MCM. He receives no personal financial payment for this work. SNF acts on behalf of University Hospital Southampton National Health Service (NHS) Foundation Trust as an investigator or providing consultative advice, or both, on clinical trials and studies of COVID-19 and other vaccines funded or sponsored by vaccine manufacturers including Janssen, Pfizer, AstraZeneca, GlaxoSmithKline, Novavax, Seqirus, Sanofi, Medimmune, Merck and Valneva. He receives no personal financial payment for this work. MR and EL, through the Immunisation Department, provide vaccine manufacturers (including Pfizer) with post-marketing surveillance reports about pneumococcal and meningococcal disease which the companies are required to submit to the UK Licensing authority in compliance with their Risk Management Strategy. A cost recovery charge is made for these reports. PA acts on behalf of the University of Oxford as the director of operations at the Oxford Vaccine Group and has received funding from the Vaccine Taskforce via the NIHR and AstraZeneca.
Figures
References
-
- Ward H, Atchison CJ, Whitaker M. Antibody prevalence for SARS-CoV-2 in England following first peak of the pandemic: REACT2 study in 100,000 adults. medRxiv 2020:2020.08.12.20173690.
-
- Mensah AA, Sinnathamby M, Zaidi A, et al. . SARS-CoV-2 infections in children following the full re-opening of schools and the impact of national lockdown: prospective, National observational cohort surveillance, July-December 2020, England. J Infect 2021;82:67–74. 10.1016/j.jinf.2021.02.022 - DOI - PMC - PubMed
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
