Investigation of CD4 and CD8 T cell-mediated protection against influenza A virus in a cohort study
- PMID: 35858844
- PMCID: PMC9301821
- DOI: 10.1186/s12916-022-02429-7
Investigation of CD4 and CD8 T cell-mediated protection against influenza A virus in a cohort study
Abstract
Background: The protective effect of T cell-mediated immunity against influenza virus infections in natural settings remains unclear, especially in seasonal epidemics.
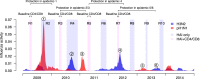
Methods: To explore the potential of such protection, we analyzed the blood samples collected longitudinally in a community-based study and covered the first wave of pandemic H1N1 (pH1N1), two subsequent pH1N1 epidemics, and three seasonal H3N2 influenza A epidemics (H3N2) for which we measured pre-existing influenza virus-specific CD4 and CD8 T cell responses by intracellular IFN-γ staining assay for 965 whole blood samples.
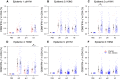
Results: Based on logistic regression, we found that higher pre-existing influenza virus-specific CD4 and CD8 T cell responses were associated with lower infection odds for corresponding subtypes. Every fold increase in H3N2-specific CD4 and CD8 T cells was associated with 28% (95% CI 8%, 44%) and 26% (95% CI 8%, 41%) lower H3N2 infection odds, respectively. Every fold increase in pre-existing seasonal H1N1 influenza A virus (sH1N1)-specific CD4 and CD8 T cells was associated with 28% (95% CI 11%, 41%) and 22% (95% CI 8%, 33%) lower pH1N1 infection odds, respectively. We observed the same associations for individuals with pre-epidemic hemagglutination inhibition (HAI) titers < 40. There was no correlation between pre-existing influenza virus-specific CD4 and CD8 T cell response and HAI titer.
Conclusions: We demonstrated homosubtypic and cross-strain protection against influenza infections was associated with T cell response, especially CD4 T cell response. These protections were independent of the protection associated with HAI titer. Therefore, T cell response could be an assessment of individual and population immunity for future epidemics and pandemics, in addition to using HAI titer.
Keywords: Influenza; Susceptibility; T cell-mediated immunity.
© 2022. The Author(s).
Conflict of interest statement
BJC reports honoraria from AstraZeneca, GlaxoSmithKline, Moderna, Roche, and Sanofi Pasteur. All other authors declare that they have no competing interests.
Figures




Similar articles
-
Cross-protective immunity against influenza pH1N1 2009 viruses induced by seasonal influenza A (H3N2) virus is mediated by virus-specific T-cells.J Gen Virol. 2011 Oct;92(Pt 10):2339-2349. doi: 10.1099/vir.0.033076-0. Epub 2011 Jun 8. J Gen Virol. 2011. PMID: 21653752
-
Human T-cells directed to seasonal influenza A virus cross-react with 2009 pandemic influenza A (H1N1) and swine-origin triple-reassortant H3N2 influenza viruses.J Gen Virol. 2013 Mar;94(Pt 3):583-592. doi: 10.1099/vir.0.048652-0. Epub 2012 Nov 14. J Gen Virol. 2013. PMID: 23152369
-
Relative incidence and individual-level severity of seasonal influenza A H3N2 compared with 2009 pandemic H1N1.BMC Infect Dis. 2017 May 11;17(1):337. doi: 10.1186/s12879-017-2432-7. BMC Infect Dis. 2017. PMID: 28494805 Free PMC article.
-
Recalling the Future: Immunological Memory Toward Unpredictable Influenza Viruses.Front Immunol. 2019 Jul 2;10:1400. doi: 10.3389/fimmu.2019.01400. eCollection 2019. Front Immunol. 2019. PMID: 31312199 Free PMC article. Review.
-
Fighting flu: novel CD8+ T-cell targets are required for future influenza vaccines.Clin Transl Immunology. 2024 Feb 14;13(2):e1491. doi: 10.1002/cti2.1491. eCollection 2024. Clin Transl Immunology. 2024. PMID: 38362528 Free PMC article. Review.
Cited by
-
Ex Pluribus Unum: The CD4 T Cell Response against Influenza A Virus.Cells. 2024 Apr 5;13(7):639. doi: 10.3390/cells13070639. Cells. 2024. PMID: 38607077 Free PMC article. Review.
-
Elucidating the critical role of gut microbiota in the pathogenesis of bacterial pneumonia: insights from a Mendelian randomization analysis mediated by immune cell.BMC Infect Dis. 2025 Mar 18;25(1):378. doi: 10.1186/s12879-025-10758-0. BMC Infect Dis. 2025. PMID: 40102744 Free PMC article.
-
Inhibition Effects of Patchouli Alcohol, Carvacrol, p-Cymene, Eucalyptol and Their Formulations Against Influenza Virus Pneumonia Through TLR4/NF-κB/NLRP3 Signaling Pathway.Chem Biol Drug Des. 2025 Aug;106(2):e70150. doi: 10.1111/cbdd.70150. Chem Biol Drug Des. 2025. PMID: 40757669 Free PMC article.
-
Lung influenza virus-specific memory CD4 T cell location and optimal cytokine production are dependent on interactions with lung antigen-presenting cells.Mucosal Immunol. 2024 Oct;17(5):843-857. doi: 10.1016/j.mucimm.2024.06.001. Epub 2024 Jun 6. Mucosal Immunol. 2024. PMID: 38851589 Free PMC article.
-
Superior antibody and membrane protein-specific T-cell responses to CoronaVac by intradermal versus intramuscular routes in adolescents.World J Pediatr. 2024 Apr;20(4):353-370. doi: 10.1007/s12519-023-00764-0. Epub 2023 Dec 12. World J Pediatr. 2024. PMID: 38085470 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

