Neuropilin 1 and its inhibitory ligand mini-tryptophanyl-tRNA synthetase inversely regulate VE-cadherin turnover and vascular permeability
- PMID: 35858913
- PMCID: PMC9300702
- DOI: 10.1038/s41467-022-31904-1
Neuropilin 1 and its inhibitory ligand mini-tryptophanyl-tRNA synthetase inversely regulate VE-cadherin turnover and vascular permeability
Abstract
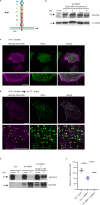
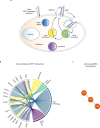
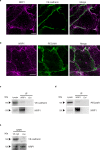
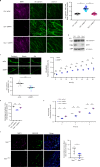
The formation of a functional blood vessel network relies on the ability of endothelial cells (ECs) to dynamically rearrange their adhesive contacts in response to blood flow and guidance cues, such as vascular endothelial growth factor-A (VEGF-A) and class 3 semaphorins (SEMA3s). Neuropilin 1 (NRP1) is essential for blood vessel development, independently of its ligands VEGF-A and SEMA3, through poorly understood mechanisms. Grounding on unbiased proteomic analysis, we report here that NRP1 acts as an endocytic chaperone primarily for adhesion receptors on the surface of unstimulated ECs. NRP1 localizes at adherens junctions (AJs) where, interacting with VE-cadherin, promotes its basal internalization-dependent turnover and favors vascular permeability initiated by histamine in both cultured ECs and mice. We identify a splice variant of tryptophanyl-tRNA synthetase (mini-WARS) as an unconventionally secreted extracellular inhibitory ligand of NRP1 that, by stabilizing it at the AJs, slows down both VE-cadherin turnover and histamine-elicited endothelial leakage. Thus, our work shows a role for NRP1 as a major regulator of AJs plasticity and reveals how mini-WARS acts as a physiological NRP1 inhibitory ligand in the control of VE-cadherin endocytic turnover and vascular permeability.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

