Development of a rapid in vitro pre-screen for distinguishing effective liposome-adjuvant delivery systems
- PMID: 35859154
- PMCID: PMC9299755
- DOI: 10.1038/s41598-022-14449-7
Development of a rapid in vitro pre-screen for distinguishing effective liposome-adjuvant delivery systems
Abstract
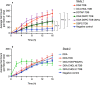
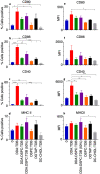
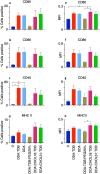
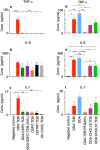
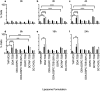
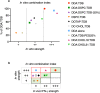
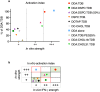
Liposomes are a strong supporting tool in vaccine technology, as they are a versatile system that not only act as antigen delivery systems but also adjuvants that can be highly effective at stimulating both innate and adaptive immune responses. Their ability to induce cell-mediated immunity makes their use in vaccines a useful tool in the development of novel, more effective vaccines against intracellular infections (e.g. HIV, malaria and tuberculosis). Currently, screening of novel liposome formulations uses murine in vivo models which generate data that often correlates poorly with human data. In addition, these models are both high cost and low throughput, making them prohibitive for large scale screening of formulation libraries. This study uses the cationic liposome formulation DDA:TDB (known as cationic adjuvant formulation 01 (CAF01)), as a lead formulation, along with other liposome formulations of known in vivo efficacy to develop an in vitro screening tool for liposome formulation development. THP-1-derived macrophages were the model antigen presenting cell used to assess the ability of the liposome formulations to attract, associate with and activate antigen presenting cells in vitro, crucial steps necessary for an effective immune response to antigen. By using a combination of in vitro functions, the study highlights the potential use of an in vitro screening tool, to predict the in vivo efficacy of novel liposome formulations. CAF01 was predicted as the most effective liposome formulation when assessing all in vitro functions and a measure of in vitro activation was able to predict 80% of the liposome correctly for their ability to induce an in vivo IFN-ү response.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

