Neurotensin orchestrates valence assignment in the amygdala
- PMID: 35859170
- PMCID: PMC9583860
- DOI: 10.1038/s41586-022-04964-y
Neurotensin orchestrates valence assignment in the amygdala
Abstract
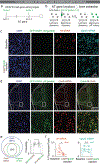
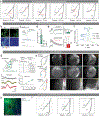
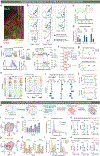
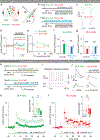
The ability to associate temporally segregated information and assign positive or negative valence to environmental cues is paramount for survival. Studies have shown that different projections from the basolateral amygdala (BLA) are potentiated following reward or punishment learning1-7. However, we do not yet understand how valence-specific information is routed to the BLA neurons with the appropriate downstream projections, nor do we understand how to reconcile the sub-second timescales of synaptic plasticity8-11 with the longer timescales separating the predictive cues from their outcomes. Here we demonstrate that neurotensin (NT)-expressing neurons in the paraventricular nucleus of the thalamus (PVT) projecting to the BLA (PVT-BLA:NT) mediate valence assignment by exerting NT concentration-dependent modulation in BLA during associative learning. We found that optogenetic activation of the PVT-BLA:NT projection promotes reward learning, whereas PVT-BLA projection-specific knockout of the NT gene (Nts) augments punishment learning. Using genetically encoded calcium and NT sensors, we further revealed that both calcium dynamics within the PVT-BLA:NT projection and NT concentrations in the BLA are enhanced after reward learning and reduced after punishment learning. Finally, we showed that CRISPR-mediated knockout of the Nts gene in the PVT-BLA pathway blunts BLA neural dynamics and attenuates the preference for active behavioural strategies to reward and punishment predictive cues. In sum, we have identified NT as a neuropeptide that signals valence in the BLA, and showed that NT is a critical neuromodulator that orchestrates positive and negative valence assignment in amygdala neurons by extending valence-specific plasticity to behaviourally relevant timescales.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
COMPETING INTEREST STATEMENT
No competing Interests.
Figures











References
-
- Shen C-J et al. Cannabinoid CB1 receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behavior. Nat. Med. 25, 337–349 (2019). - PubMed
MeSH terms
Substances
Grants and funding
- K99 MH124435/MH/NIMH NIH HHS/United States
- F32 MH111216/MH/NIMH NIH HHS/United States
- R00 MH124435/MH/NIMH NIH HHS/United States
- R37 MH102441/MH/NIMH NIH HHS/United States
- T32 NS007292/NS/NINDS NIH HHS/United States
- P50 MH115874/MH/NIMH NIH HHS/United States
- K99 DA055111/DA/NIDA NIH HHS/United States
- R01 MH108665/MH/NIMH NIH HHS/United States
- DP1 AT009925/AT/NCCIH NIH HHS/United States
- T32 GM133351/GM/NIGMS NIH HHS/United States
- F32 MH115446/MH/NIMH NIH HHS/United States
- DP2 DK102256/DK/NIDDK NIH HHS/United States
- R01 MH102441/MH/NIMH NIH HHS/United States
- R00 DA045103/DA/NIDA NIH HHS/United States
- K99 DA045103/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

