Oocytes maintain ROS-free mitochondrial metabolism by suppressing complex I
- PMID: 35859172
- PMCID: PMC9329100
- DOI: 10.1038/s41586-022-04979-5
Oocytes maintain ROS-free mitochondrial metabolism by suppressing complex I
Abstract
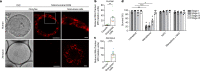
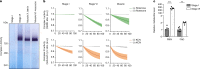
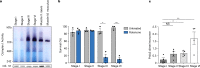
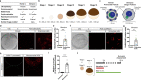
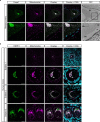
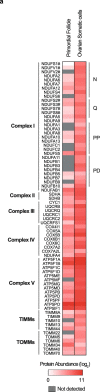
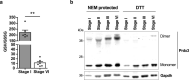
Oocytes form before birth and remain viable for several decades before fertilization1. Although poor oocyte quality accounts for most female fertility problems, little is known about how oocytes maintain cellular fitness, or why their quality eventually declines with age2. Reactive oxygen species (ROS) produced as by-products of mitochondrial activity are associated with lower rates of fertilization and embryo survival3-5. Yet, how healthy oocytes balance essential mitochondrial activity with the production of ROS is unknown. Here we show that oocytes evade ROS by remodelling the mitochondrial electron transport chain through elimination of complex I. Combining live-cell imaging and proteomics in human and Xenopus oocytes, we find that early oocytes exhibit greatly reduced levels of complex I. This is accompanied by a highly active mitochondrial unfolded protein response, which is indicative of an imbalanced electron transport chain. Biochemical and functional assays confirm that complex I is neither assembled nor active in early oocytes. Thus, we report a physiological cell type without complex I in animals. Our findings also clarify why patients with complex-I-related hereditary mitochondrial diseases do not experience subfertility. Complex I suppression represents an evolutionarily conserved strategy that allows longevity while maintaining biological activity in long-lived oocytes.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Comment in
-
Eggs remodel energy production to protect themselves from harm.Nature. 2022 Jul;607(7920):664-665. doi: 10.1038/d41586-022-01642-x. Nature. 2022. PMID: 35859121 No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

