Synthesis of bis-spirocyclic derivatives of 3-azabicyclo[3.1.0]hexane via cyclopropene cycloadditions to the stable azomethine ylide derived from Ruhemann's purple
- PMID: 35859623
- PMCID: PMC9263550
- DOI: 10.3762/bjoc.18.77
Synthesis of bis-spirocyclic derivatives of 3-azabicyclo[3.1.0]hexane via cyclopropene cycloadditions to the stable azomethine ylide derived from Ruhemann's purple
Abstract
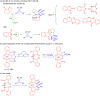
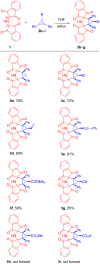
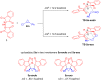
A reliable method for the synthesis of bis-spirocyclic derivatives of 3-azabicyclo[3.1.0]hexanes through the 1,3-dipolar cycloaddition (1,3-DC) reactions of cyclopropenes to the stable azomethine ylide - protonated form of Ruhemann's purple (PRP) has been developed. Both 3-substituted and 3,3-disubstituted cyclopropenes reacted with PRP, affording the corresponding bis-spirocyclic 3-azabicyclo[3.1.0]hexane cycloadducts in moderate to good yields with high diastereofacial selectivity. Moreover, several unstable 1,2-disubstituted cyclopropenes were successfully trapped by the stable 1,3-dipole under mild conditions. The mechanism of the cycloaddition reactions of cyclopropenes with PRP has been thoroughly studied using density functional theory (DFT) methods at the M11/cc-pVDZ level of theory. The cycloaddition reactions have been found to be HOMOcyclopropene-LUMOylide controlled while the transition-state energies for the reaction of 3-methyl-3-phenylcyclopropene with PRP are fully consistent with the experimentally observed stereoselectivity.
Keywords: DFT calculations; azomethine ylides; cycloaddition; cyclopropenes; spiro heterocycles.
Copyright © 2022, Filatov et al.
Figures



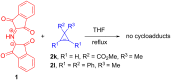



References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
