Novel Thioxothiazolo[3,4- a]quinazolin-5(4 H)-one Derivatives as BKCa Channel Activators for Urinary Incontinence
- PMID: 35859863
- PMCID: PMC9290006
- DOI: 10.1021/acsmedchemlett.2c00070
Novel Thioxothiazolo[3,4- a]quinazolin-5(4 H)-one Derivatives as BKCa Channel Activators for Urinary Incontinence
Abstract
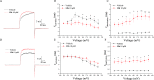
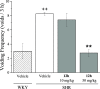
Overactive bladder (OAB) is a syndrome causing a sudden and unstoppable need to urinate with significant global prevalence. Several drugs are used to treat OAB; however, they have various side effects. Therefore, new treatment options for OAB are required. A series of novel 5-oxo-N-phenyl-1-thioxo-4,5-dihydro-1H-thiazolo[3,4-a]quinazoline-3-carboxamide derivatives were synthesized and evaluated for their large-conductance voltage- and Ca2+-activated K+ channel activation through a cell-based fluorescence assay and electrophysiological recordings. Several compounds, including a 7-bromo substituent on the heterocyclic system, showed increased channel currents. Among the derivatives, compound 12h exhibited potent in vitro activity with a half-maximal effective concentration (EC50) of 2.89 μM, good oral pharmacokinetic properties (area under the curve and half-life), and in vivo efficacy in a spontaneously hypertensive rat model.
© 2022 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous

