Lysophosphatidylcholine Alleviates Acute Lung Injury by Regulating Neutrophil Motility and Neutrophil Extracellular Trap Formation
- PMID: 35859904
- PMCID: PMC9289271
- DOI: 10.3389/fcell.2022.941914
Lysophosphatidylcholine Alleviates Acute Lung Injury by Regulating Neutrophil Motility and Neutrophil Extracellular Trap Formation
Abstract
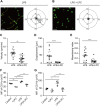
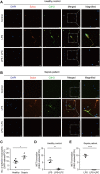
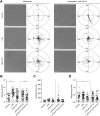
Sepsis is predominantly initiated by bacterial infection and can cause systemic inflammation, which frequently leads to rapid death of the patient. However, this acute systemic inflammatory response requires further investigation from the perspectives of clinical judgment criteria and early treatment strategies for the relief of symptoms. Lysophosphatidylcholine (LPC) 18:0 may relieve septic symptoms, but the relevant mechanism is not clearly understood. Therefore, we aimed to assess the effectiveness of LPC as a therapeutic treatment for acute inflammation in the lung induced by lipopolysaccharide in mice. Systemic inflammation of mice was induced by lipopolysaccharide (LPS) inoculation to investigate the role of LPC in the migration and the immune response of neutrophils during acute lung injury. By employing two-photon intravital imaging of the LPS-stimulated LysM-GFP mice and other in vitro and in vivo assays, we examined whether LPC alleviates the inflammatory effect of sepsis. We also tested the effect of LPC to human neutrophils from healthy control and sepsis patients. Our data showed that LPC treatment reduced the infiltration of innate immune cells into the lung. Specifically, LPC altered neutrophil migratory patterns and enhanced phagocytic efficacy in the damaged lung. Moreover, LPC treatment reduced the release of neutrophil extracellular trap (NET), which can damage tissue in the inflamed organ and exacerbate disease. It also reduced human neutrophil migration under inflammatory environment. Our results suggest that LPC can alleviate sepsis-induced lung inflammation by regulating the function of neutrophils. These findings provide evidence for the beneficial application of LPC treatment as a potential therapeutic strategy for sepsis.
Keywords: NET; imaging; inflammation; lysophosphatidylcholine; sepsis.
Copyright © 2022 Jeong, Kim, Byun, Jin, Seo, Shin, Leem, Choung, Park and Hyun.
Conflict of interest statement
Authors SJi, BS, JC are employed by Aribio Co. LTD. The remaining authors declare that the research was conducted by in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures




Similar articles
-
Lysophosphatidylcholine modulates neutrophil oxidant production through elevation of cyclic AMP.J Immunol. 2005 Mar 1;174(5):2981-9. doi: 10.4049/jimmunol.174.5.2981. J Immunol. 2005. PMID: 15728511
-
In vivo monitoring of dynamic interaction between neutrophil and human umbilical cord blood-derived mesenchymal stem cell in mouse liver during sepsis.Stem Cell Res Ther. 2020 Feb 3;11(1):44. doi: 10.1186/s13287-020-1559-4. Stem Cell Res Ther. 2020. PMID: 32014040 Free PMC article.
-
Neutralization of osteopontin attenuates neutrophil migration in sepsis-induced acute lung injury.Crit Care. 2015 Feb 26;19(1):53. doi: 10.1186/s13054-015-0782-3. Crit Care. 2015. PMID: 25887405 Free PMC article.
-
Real-time observation of neutrophil extracellular trap formation in the inflamed mouse brain via two-photon intravital imaging.Lab Anim Res. 2022 Jun 13;38(1):16. doi: 10.1186/s42826-022-00126-3. Lab Anim Res. 2022. PMID: 35698178 Free PMC article. Review.
-
Autophagy-driven neutrophil extracellular traps: The dawn of sepsis.Pathol Res Pract. 2022 Jun;234:153896. doi: 10.1016/j.prp.2022.153896. Epub 2022 Apr 18. Pathol Res Pract. 2022. PMID: 35462228 Review.
Cited by
-
NLRP3 exacerbates EAE severity through ROS-dependent NET formation in the mouse brain.Cell Commun Signal. 2024 Feb 2;22(1):96. doi: 10.1186/s12964-023-01447-z. Cell Commun Signal. 2024. PMID: 38308301 Free PMC article.
-
NLRP3 Exacerbate NETosis-Associated Neuroinflammation in an LPS-Induced Inflamed Brain.Immune Netw. 2023 May 8;23(3):e27. doi: 10.4110/in.2023.23.e27. eCollection 2023 Jun. Immune Netw. 2023. PMID: 37416934 Free PMC article.
-
Lysophosphatidylcholine 14:0 Alleviates Lipopolysaccharide-Induced Acute Lung Injury via Protecting Alveolar Epithelial Barrier by Activation of Nrf2/HO-1 Pathway.J Inflamm Res. 2024 Dec 6;17:10533-10546. doi: 10.2147/JIR.S495227. eCollection 2024. J Inflamm Res. 2024. PMID: 39659750 Free PMC article.
-
Combining lipidomics and machine learning to identify lipid biomarkers for nonsyndromic cleft lip with palate.JCI Insight. 2025 May 8;10(9):e186629. doi: 10.1172/jci.insight.186629. eCollection 2025 May 8. JCI Insight. 2025. PMID: 40337862 Free PMC article.
-
Identifying early predictive and diagnostic biomarkers and exploring metabolic pathways for sepsis after trauma based on an untargeted metabolomics approach.Sci Rep. 2025 Apr 8;15(1):12068. doi: 10.1038/s41598-025-92631-3. Sci Rep. 2025. PMID: 40199964 Free PMC article.
References
-
- Ahn S. Y., Maeng Y.-S., Kim Y. R., Choe Y. H., Hwang H. S., Hyun Y.-M. (2020). In Vivo monitoring of Dynamic Interaction between Neutrophil and Human Umbilical Cord Blood-Derived Mesenchymal Stem Cell in Mouse Liver during Sepsis. Stem Cell. Res. Ther. 11, 44. 10.1186/s13287-020-1559-4 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

