Development of STEAP1 targeting chimeric antigen receptor for adoptive cell therapy against cancer
- PMID: 35860008
- PMCID: PMC9278049
- DOI: 10.1016/j.omto.2022.06.007
Development of STEAP1 targeting chimeric antigen receptor for adoptive cell therapy against cancer
Abstract
Chimeric antigen receptors (CARs) that retarget T cells against CD19 show clinical efficacy against B cell malignancies. Here, we describe the development of a CAR against the six-transmembrane epithelial antigen of prostate-1 (STEAP1), which is expressed in ∼90% of prostate cancers, and subgroups of other malignancies. STEAP1 is an attractive target, as it is associated with tumor invasiveness and progression and only expressed at low levels in normal tissues, apart from the non-vital prostate gland. We identified the antibody coding sequences from a hybridoma and designed a CAR that is efficiently expressed in primary T cells. The T cells acquired the desired anti-STEAP1 specificity, with a polyfunctional response including production of multiple cytokines, proliferation, and the killing of cancer cells. The response was observed for both CD4+ and CD8+ T cells, and against all STEAP1+ target cell lines tested. We evaluated the in vivo CAR T activity in both subcutaneous and metastatic xenograft mouse models of prostate cancer. Here, the CAR T cells infiltrated tumors and significantly inhibited tumor growth and extended survival in a STEAP1-dependent manner. We conclude that the STEAP1 CAR exhibits potent in vitro and in vivo functionality and can be further developed toward potential clinical use.
Keywords: CAR T cell; STEAP1; cancer immunotherapy; cell therapy; chimeric antigen receptor; metastatic cancer mouse model; prostate cancer.
© 2022 The Author(s).
Conflict of interest statement
J.A.K. and Yixin Jin are inventors on a patent application related to the work described in this article.
Figures

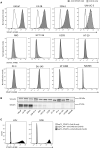


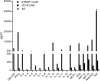


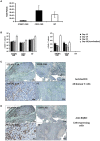

Similar articles
-
Development of a TGFβ-IL-2/15 Switch Receptor for Use in Adoptive Cell Therapy.Biomedicines. 2023 Feb 4;11(2):459. doi: 10.3390/biomedicines11020459. Biomedicines. 2023. PMID: 36830995 Free PMC article.
-
Comparative Evaluation of STEAP1 Targeting Chimeric Antigen Receptors with Different Costimulatory Domains and Spacers.Int J Mol Sci. 2024 Jan 2;25(1):586. doi: 10.3390/ijms25010586. Int J Mol Sci. 2024. PMID: 38203757 Free PMC article.
-
Targeting advanced prostate cancer with STEAP1 chimeric antigen receptor T cell and tumor-localized IL-12 immunotherapy.Nat Commun. 2023 Apr 11;14(1):2041. doi: 10.1038/s41467-023-37874-2. Nat Commun. 2023. PMID: 37041154 Free PMC article.
-
Targeting STEAP1 as an anticancer strategy.Front Oncol. 2023 Oct 16;13:1285661. doi: 10.3389/fonc.2023.1285661. eCollection 2023. Front Oncol. 2023. PMID: 37909017 Free PMC article. Review.
-
The Role of STEAP1 in Prostate Cancer: Implications for Diagnosis and Therapeutic Strategies.Biomedicines. 2025 Mar 26;13(4):794. doi: 10.3390/biomedicines13040794. Biomedicines. 2025. PMID: 40299363 Free PMC article. Review.
Cited by
-
Development of a TGFβ-IL-2/15 Switch Receptor for Use in Adoptive Cell Therapy.Biomedicines. 2023 Feb 4;11(2):459. doi: 10.3390/biomedicines11020459. Biomedicines. 2023. PMID: 36830995 Free PMC article.
-
CAR-Based Immunotherapy of Solid Tumours-A Survey of the Emerging Targets.Cancers (Basel). 2023 Feb 11;15(4):1171. doi: 10.3390/cancers15041171. Cancers (Basel). 2023. PMID: 36831514 Free PMC article. Review.
-
Exploring STEAP1 Expression in Prostate Cancer Cells in Response to Androgen Deprivation and in Small Extracellular Vesicles.Mol Cancer Res. 2025 Jun 3;23(6):542-552. doi: 10.1158/1541-7786.MCR-24-0903. Mol Cancer Res. 2025. PMID: 40287951
-
Development of a High-Affinity Antibody against the Tumor-Specific and Hyperactive 611-p95HER2 Isoform.Cancers (Basel). 2022 Oct 5;14(19):4859. doi: 10.3390/cancers14194859. Cancers (Basel). 2022. PMID: 36230782 Free PMC article.
-
Targeting Aggressive Prostate Carcinoma Cells with Mesothelin-CAR-T Cells.Biomedicines. 2025 May 16;13(5):1215. doi: 10.3390/biomedicines13051215. Biomedicines. 2025. PMID: 40427042 Free PMC article.
References
-
- Brentjens R.J., Davila M.L., Riviere I., Park J., Wang X., Cowell L.G., Bartido S., Stefanski J., Taylor C., Olszewska M., et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 2013;5:177ra38. doi: 10.1126/scitranslmed.3005930. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

