Hepatitis B Virus Reactivation and Mycobacterial Infections Associated With Ustekinumab: A Retrospective Study of an International Pharmacovigilance Database
- PMID: 35860015
- PMCID: PMC9289361
- DOI: 10.3389/fphar.2022.921084
Hepatitis B Virus Reactivation and Mycobacterial Infections Associated With Ustekinumab: A Retrospective Study of an International Pharmacovigilance Database
Abstract
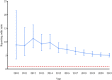
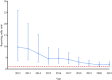
Background: Reports were recently published on hepatitis B virus reactivation (HBVr), tuberculosis (TB), and atypical mycobacterial infection (AMI) in patients with ustekinumab treatment. However, the literature is limited to case reports and series. The study was aimed to investigate their relationships by using an extensive population-based database. Methods: Using the United States Food and Drug Administration Adverse Event Reporting System (FAERS) database, we collected all cases of HBVr, TB, and AMI between 1 January 2009 and 30 September 2021, for ustekinumab and other drugs. Disproportionality was analyzed using the reporting odds ratio (ROR), which was considered significant when the lower limit of the 95% confidence interval (95% CI) was >1. Results: Of the 18,760,438 adverse cases reported to FAERS for all drugs, 56,581 cases had been exposed to ustekinumab. Adverse events of HBVr, TB, and AMI were reported in 21, 210, and 20 cases, respectively. The ROR for HBVr with ustekinumab was 2.33 (95% CI, 1.52-3.58), for TB was 5.09 (95% CI, 4.44-5.84), and for AMI was 2.09 (95% CI, 1.35-3.24). In the ustekinumab exposure group, no death occurred in patients with HBVr, but one patient experienced life-threatening liver failure. For those with TB, 24 cases experienced hospitalization and 2 deaths occurred. No death occurred in patients with AMI but eight experienced hospitalization. Conclusion: We identified positive signals between ustekinumab exposure and HBVr, TB, and AMI in FAERS. Although these complications are rare, clinicians using ustekinumab should be aware of the risks.
Keywords: atypical mycobacterial infection; hepatitis B; pharmacovigilance; tuberculosis; ustekinumab.
Copyright © 2022 Wang, Geng, Zhang, Xiao and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Risk of hepatitis B virus reactivation following treatment with abatacept: A retrospective study of international pharmacovigilance databases.EClinicalMedicine. 2022 May 5;48:101425. doi: 10.1016/j.eclinm.2022.101425. eCollection 2022 Jun. EClinicalMedicine. 2022. PMID: 35706497 Free PMC article.
-
Hepatitis B virus reactivation associated with Janus kinase (JAK) inhibitors: a retrospective study of pharmacovigilance databases and review of the literature.Expert Opin Drug Saf. 2023 Jan-Jun;22(6):469-476. doi: 10.1080/14740338.2023.2181339. Epub 2023 Feb 21. Expert Opin Drug Saf. 2023. PMID: 36794347 Review.
-
Mycobacterial Infections With Ruxolitinib: A Retrospective Pharmacovigilance Review.Clin Lymphoma Myeloma Leuk. 2020 Jan;20(1):18-23. doi: 10.1016/j.clml.2019.08.008. Epub 2019 Aug 26. Clin Lymphoma Myeloma Leuk. 2020. PMID: 31699655 Review.
-
Mycobacterial infections due to PD-1 and PD-L1 checkpoint inhibitors.ESMO Open. 2020 Aug;5(4):e000866. doi: 10.1136/esmoopen-2020-000866. ESMO Open. 2020. PMID: 32817069 Free PMC article.
-
Real-World Pharmacovigilance Study Identifies Drugs Linked to Hepatitis B Virus Reactivation.J Med Virol. 2024 Nov;96(11):e70055. doi: 10.1002/jmv.70055. J Med Virol. 2024. PMID: 39558790
Cited by
-
Real-world individual and comparative analysis of adverse event reporting for adalimumab and etanercept using public FDA adverse event reporting system data.Arch Dermatol Res. 2024 Dec 30;317(1):161. doi: 10.1007/s00403-024-03626-5. Arch Dermatol Res. 2024. PMID: 39738670
References
-
- Benson J. M., Peritt D., Scallon B. J., Heavner G. A., Shealy D. J., Giles-Komar J. M., et al. (2011). Discovery and Mechanism of Ustekinumab: a Human Monoclonal Antibody Targeting Interleukin-12 and Interleukin-23 for Treatment of Immune-Mediated Disorders. MAbs 3, 535–545. 10.4161/mabs.3.6.17815 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

