Effect of Algoplaque Hydrocolloid Dressing Combined with Nanosilver Antibacterial Gel under Predictive Nursing in the Treatment of Medical Device-Related Pressure Injury
- PMID: 35860183
- PMCID: PMC9293497
- DOI: 10.1155/2022/9756602
Effect of Algoplaque Hydrocolloid Dressing Combined with Nanosilver Antibacterial Gel under Predictive Nursing in the Treatment of Medical Device-Related Pressure Injury
Abstract
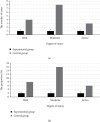
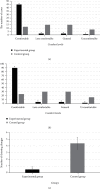
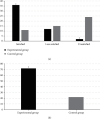
It was aimed at the clinical value of predictive nursing and Algoplaque hydrocolloid dressing (AHD) combined with nanosilver antibacterial gel in treating medical device-related pressure injury (MDRPI). 100 patients, who underwent surgery in Chongqing Qijiang District People's Hospital from February 2019 to February 2020, were selected as the research objects and were randomly divided into the experimental group (50 cases) and the control group (50 cases). For the characterization test, a nanosilver antibacterial gel was created first. Patients in both groups received predictive nursing, but those in the experimental group received AHD and nanosilver antibacterial gel, and those in the control group received gauzes. MDRPI incidence, pressed skin injury severity, comfort level, clothing changes, nursing satisfaction, and other factors were all compared. The particle size of the nanosilver gel was 45-85 nm, with a relatively homogeneous distribution with the medium size, according to the findings. The incidence of MDRPI in the experimental group was lower than that in the control group significantly (6% vs. 30%, P < 0.05). The degree of injury of pressured skin in the experimental group was milder than that in the control group (P < 0.05), the degree of comfort and nursing satisfaction was higher in the experimental group than in the control group (P < 0.05), and dressing change count was lower than that in the control group (P < 0.05). In the treatment of MDRPI, predictive nursing and AHD using nanosilver antibacterial gel showed high clinical application value.
Copyright © 2022 Chunxiu Li et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
-
- Lustig A., Margi R., Orlov A., Orlova D., Azaria L., Gefen A. The mechanobiology theory of the development of medical device-related pressure ulcers revealed through a cell-scale computational modeling framework. Biomechanics and Modeling in Mechanobiology . 2021;20(3):851–860. doi: 10.1007/s10237-021-01432-w. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

