Colchicine Impacts Leukocyte Trafficking in Atherosclerosis and Reduces Vascular Inflammation
- PMID: 35860249
- PMCID: PMC9289246
- DOI: 10.3389/fimmu.2022.898690
Colchicine Impacts Leukocyte Trafficking in Atherosclerosis and Reduces Vascular Inflammation
Abstract
Background: Inflammation strongly contributes to atherosclerosis initiation and progression. Consequently, recent clinical trials pharmacologically targeted vascular inflammation to decrease the incidence of atherosclerosis-related complications. Colchicine, a microtubule inhibitor with anti-inflammatory properties, reduced cardiovascular events in patients with recent acute coronary syndrome and chronic coronary disease. However, the biological basis of these observations remains elusive. We sought to explore the mechanism by which colchicine beneficially alters the course of atherosclerosis.
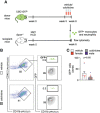
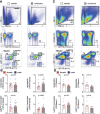
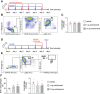
Methods and results: In mice with early atherosclerosis (Apoe-/- mice on a high cholesterol diet for 8 weeks), we found that colchicine treatment (0.25 mg/kg bodyweight once daily over four weeks) reduced numbers of neutrophils, inflammatory monocytes and macrophages inside atherosclerotic aortas using flow cytometry and immunohistochemistry. Consequently, colchicine treatment resulted in a less inflammatory plaque composition and reduced plaque size. We next investigated how colchicine prevented plaque leukocyte expansion and found that colchicine treatment mitigated recruitment of blood neutrophils and inflammatory monocytes to plaques as revealed by adoptive transfer experiments. Causally, we found that colchicine reduced levels of both leukocyte adhesion molecules and receptors for leukocyte chemoattractants on blood neutrophils and monocytes. Further experiments showed that colchicine treatment reduced vascular inflammation also in post-myocardial infarction accelerated atherosclerosis through similar mechanisms as documented in early atherosclerosis. When we examined whether colchicine also decreased numbers of macrophages inside atherosclerotic plaques by impacting monocyte/macrophage transitioning or in-situ proliferation of macrophages, we report that colchicine treatment did not influence macrophage precursor differentiation or macrophage proliferation using cell culture experiments with bone marrow derived macrophages.
Conclusions: Our data reveal that colchicine prevents expansion of plaque inflammatory leukocytes through lowering recruitment of blood myeloid cells to plaques. These data provide novel mechanistic clues on the beneficial effects of colchicine in the treatment of atherosclerosis and may inform future anti-inflammatory interventions in patients at risk.
Keywords: anti-inflammatory treatment; atherosclerosis; colchicine; innate immunity; leukocyte recruitment; monocytes/macrophages; neutrophils; vascular inflammation.
Copyright © 2022 Meyer-Lindemann, Mauersberger, Schmidt, Moggio, Hinterdobler, Li, Khangholi, Hettwer, Gräßer, Dutsch, Schunkert, Kessler and Sager.
Conflict of interest statement
HSa reports grants from the European Research Council, the “Else-Kröner-Fresenius-Stiftung”, the “Deutsche Herzstiftung” and the “Deutsche Forschungsgemeinschaft” during the conduct of the study. HSa received lecture fees from Novo Nordisk. HSc reports personal fees from MSD Sharp & Dohme, personal fees from AMGEN, personal fees from Bayer Vital GmbH, personal fees from Boehringer Ingelheim, personal fees from Daiichi-Sankyo, personal fees from Novartis, personal fees from Servier, personal fees from Brahms, personal fees from Bristol-Myers Squibb, personal fees from Medtronic, personal fees from Sanofi Aventis, personal fees from Synlab, personal fees from Pfizer, grants and personal fees from Astra-Zeneca and personal fees from Vifor, outside the submitted work. HSc and TK are named inventors on a patent application for prevention of restenosis after angioplasty and stent implantation outside the submitted work. TK received lecture fees from Bayer AG, Pharmaceuticals. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Interleukin-1β suppression dampens inflammatory leucocyte production and uptake in atherosclerosis.Cardiovasc Res. 2022 Oct 21;118(13):2778-2791. doi: 10.1093/cvr/cvab337. Cardiovasc Res. 2022. PMID: 34718444 Free PMC article.
-
The Beneficial Therapy with Colchicine for Atherosclerosis via Anti-inflammation and Decrease in Hypertriglyceridemia.Cardiovasc Hematol Agents Med Chem. 2018;16(2):74-80. doi: 10.2174/1871525717666181211110332. Cardiovasc Hematol Agents Med Chem. 2018. PMID: 30526472 Review.
-
The anti-inflammatory vasostatin-2 attenuates atherosclerosis in ApoE-/- mice and inhibits monocyte/macrophage recruitment.Thromb Haemost. 2017 Jan 26;117(2):401-414. doi: 10.1160/TH16-06-0475. Epub 2016 Nov 10. Thromb Haemost. 2017. PMID: 27831589
-
Insight into the Mechanistic role of Colchicine in Atherosclerosis.Curr Atheroscler Rep. 2025 Mar 20;27(1):40. doi: 10.1007/s11883-025-01291-1. Curr Atheroscler Rep. 2025. PMID: 40111634 Review.
-
P2Y6 deficiency limits vascular inflammation and atherosclerosis in mice.Arterioscler Thromb Vasc Biol. 2014 Oct;34(10):2237-45. doi: 10.1161/ATVBAHA.114.303585. Epub 2014 Aug 7. Arterioscler Thromb Vasc Biol. 2014. PMID: 25104800
Cited by
-
Colchicine prevents oxidative stress-induced endothelial cell senescence via blocking NF-κB and MAPKs: implications in vascular diseases.J Inflamm (Lond). 2023 Nov 24;20(1):41. doi: 10.1186/s12950-023-00366-7. J Inflamm (Lond). 2023. PMID: 38001470 Free PMC article.
-
Long-Lasting Recovery From Recurrent Brain Radionecrosis Following TRICO Treatment in 2 Head and Neck Cancer Survivors.Adv Radiat Oncol. 2025 Apr 7;10(6):101785. doi: 10.1016/j.adro.2025.101785. eCollection 2025 Jun. Adv Radiat Oncol. 2025. PMID: 40486288 Free PMC article. No abstract available.
-
Suspected colchicine-induced late myocardial rupture occurring after the late presentation of acute inferior ST-elevation myocardial infarction.Am J Cardiovasc Dis. 2024 Apr 15;14(2):116-120. doi: 10.62347/FXLN8938. eCollection 2024. Am J Cardiovasc Dis. 2024. PMID: 38764546 Free PMC article.
-
The Impact of Exercise on Immunity, Metabolism, and Atherosclerosis.Int J Mol Sci. 2023 Feb 8;24(4):3394. doi: 10.3390/ijms24043394. Int J Mol Sci. 2023. PMID: 36834808 Free PMC article. Review.
-
Colchicine prevents accelerated atherosclerosis in TET2-mutant clonal haematopoiesis.Eur Heart J. 2024 Nov 14;45(43):4601-4615. doi: 10.1093/eurheartj/ehae546. Eur Heart J. 2024. PMID: 39212933 Free PMC article.
References
-
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory Therapy With Canakinumab for Atherosclerotic Disease. New Engl J Med (2017) 377:1119–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

