The Hepatoprotective and Hepatotoxic Roles of Sex and Sex-Related Hormones
- PMID: 35860276
- PMCID: PMC9289199
- DOI: 10.3389/fimmu.2022.939631
The Hepatoprotective and Hepatotoxic Roles of Sex and Sex-Related Hormones
Abstract
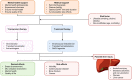
Most liver diseases, including acute liver injury, drug-induced liver injury, viral hepatitis, metabolic liver diseases, and end-stage liver diseases, are strongly linked with hormonal influences. Thus, delineating the clinical manifestation and underlying mechanisms of the "sexual dimorphism" is critical for providing hints for the prevention, management, and treatment of those diseases. Whether the sex hormones (androgen, estrogen, and progesterone) and sex-related hormones (gonadotrophin-releasing hormone, luteinizing hormone, follicle-stimulating hormone, and prolactin) play protective or toxic roles in the liver depends on the biological sex, disease stage, precipitating factor, and even the psychiatric status. Lifestyle factors, such as obesity, alcohol drinking, and smoking, also drastically affect the involving mechanisms of those hormones in liver diseases. Hormones deliver their hepatic regulatory signals primarily via classical and non-classical receptors in different liver cell types. Exogenous sex/sex-related hormone therapy may serve as a novel strategy for metabolic liver disease, cirrhosis, and liver cancer. However, the undesired hormone-induced liver injury should be carefully studied in pre-clinical models and monitored in clinical applications. This issue is particularly important for menopause females with hormone replacement therapy (HRT) and transgender populations who want to receive gender-affirming hormone therapy (GAHT). In conclusion, basic and clinical studies are warranted to depict the detailed hepatoprotective and hepatotoxic mechanisms of sex/sex-related hormones in liver disease. Prolactin holds a promising perspective in treating metabolic and advanced liver diseases.
Keywords: chronic liver diseases; cirrhosis; mechanism; sex hormone; therapy.
Copyright © 2022 Xu, Yuan, Che, Tan, Wu, Wang, Xu and Xiao.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
A study of the endocrine manifestations of hepatic cirrhosis.Q J Med. 1976 Jan;45(177):145-78. Q J Med. 1976. PMID: 769039
-
[Effect of the steroid sex hormones on the LH and FSH responses to LHRH in the normal subject].Pathol Biol (Paris). 1975 Dec;23(10):917-22. Pathol Biol (Paris). 1975. PMID: 772543 French.
-
Relation between sex hormones and hepatocellular carcinoma.Andrologia. 2016 Nov;48(9):948-955. doi: 10.1111/and.12536. Epub 2016 Jan 21. Andrologia. 2016. PMID: 26791111
-
Molecular mechanisms of hormones implicated in migraine and the translational implication for transgender patients.Front Pain Res (Lausanne). 2023 Sep 19;4:1117842. doi: 10.3389/fpain.2023.1117842. eCollection 2023. Front Pain Res (Lausanne). 2023. PMID: 37795389 Free PMC article. Review.
-
Role of sex in liver tumor occurrence and clinical outcomes: A comprehensive review.Hepatology. 2024 May 1;79(5):1141-1157. doi: 10.1097/HEP.0000000000000277. Epub 2023 Jan 3. Hepatology. 2024. PMID: 37013373 Review.
Cited by
-
Estrogen-related genes influence immune cell infiltration and immunotherapy response in Hepatocellular Carcinoma.Front Immunol. 2023 Feb 6;14:1114717. doi: 10.3389/fimmu.2023.1114717. eCollection 2023. Front Immunol. 2023. PMID: 36814910 Free PMC article.
-
MASLD: Prevalence, Mechanisms, and Sex-Based Therapies in Postmenopausal Women.Biomedicines. 2025 Apr 2;13(4):855. doi: 10.3390/biomedicines13040855. Biomedicines. 2025. PMID: 40299427 Free PMC article. Review.
-
Increased visceral fat area to skeletal muscle mass ratio is positively associated with the risk of cardiometabolic diseases in a Chinese natural population: A cross-sectional study.Diabetes Metab Res Rev. 2023 Feb;39(2):e3597. doi: 10.1002/dmrr.3597. Epub 2022 Dec 2. Diabetes Metab Res Rev. 2023. PMID: 36426681 Free PMC article.
-
Liver Microenvironment Response to Prostate Cancer Metastasis and Hormonal Therapy.Cancers (Basel). 2022 Dec 15;14(24):6189. doi: 10.3390/cancers14246189. Cancers (Basel). 2022. PMID: 36551674 Free PMC article. Review.
-
Proteomic Analysis of the Murine Liver Response to Oral Exposure to Aflatoxin B1 and Ochratoxin A: The Protective Role to Bioactive Compounds.Toxins (Basel). 2025 Jan 9;17(1):29. doi: 10.3390/toxins17010029. Toxins (Basel). 2025. PMID: 39852982 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

