SARS-CoV-2 Spike- and Nucleoprotein-Specific Antibodies Induced After Vaccination or Infection Promote Classical Complement Activation
- PMID: 35860286
- PMCID: PMC9289266
- DOI: 10.3389/fimmu.2022.838780
SARS-CoV-2 Spike- and Nucleoprotein-Specific Antibodies Induced After Vaccination or Infection Promote Classical Complement Activation
Abstract
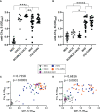
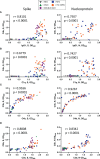
Antibodies specific for the spike glycoprotein (S) and nucleocapsid (N) SARS-CoV-2 proteins are typically present during severe COVID-19, and induced to S after vaccination. The binding of viral antigens by antibody can initiate the classical complement pathway. Since complement could play pathological or protective roles at distinct times during SARS-CoV-2 infection we determined levels of antibody-dependent complement activation along the complement cascade. Here, we used an ELISA assay to assess complement protein binding (C1q) and the deposition of C4b, C3b, and C5b to S and N antigens in the presence of antibodies to SARS-CoV-2 from different test groups: non-infected, single and double vaccinees, non-hospitalised convalescent (NHC) COVID-19 patients and convalescent hospitalised (ITU-CONV) COVID-19 patients. C1q binding correlates strongly with antibody responses, especially IgG1 levels. However, detection of downstream complement components, C4b, C3b and C5b shows some variability associated with the subject group from whom the sera were obtained. In the ITU-CONV, detection of C3b-C5b to S was observed consistently, but this was not the case in the NHC group. This is in contrast to responses to N, where median levels of complement deposition did not differ between the NHC and ITU-CONV groups. Moreover, for S but not N, downstream complement components were only detected in sera with higher IgG1 levels. Therefore, the classical pathway is activated by antibodies to multiple SARS-CoV-2 antigens, but the downstream effects of this activation may differ depending the disease status of the subject and on the specific antigen targeted.
Keywords: COVID-19; SARS-CoV-2; antibodies; complement; vaccine.
Copyright © 2022 Lamerton, Marcial-Juarez, Faustini, Perez-Toledo, Goodall, Jossi, Newby, Chapple, Dietrich, Veenith, Shields, Harper, Henderson, Rayes, Wraith, Watson, Crispin, Drayson, Richter and Cunningham.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20133 UK Patients in Hospital With Covid-19 Using the ISARIC WHO Clinical Characterisation Protocol: Prospective Observational Cohort Study. BMJ-British Med J (2020) 369:12. doi: 10.1136/bmj.m1985 - DOI - PMC - PubMed
-
- Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors Associated With Hospital Admission and Critical Illness Among 5279 People With Coronavirus Disease 2019 in New York City: Prospective Cohort Study. BMJ-British Med J (2020) 369:15. doi: 10.1136/bmj.m1966 - DOI - PMC - PubMed
-
- Shields AM, Faustini SE, Perez-Toledo M, Jossi S, Allen JD, Al-Taei S, et al. Serological Responses to SARS-CoV-2 Following non-Hospitalised Infection: Clinical and Ethnodemographic Features Associated With the Magnitude of the Antibody Response. BMJ Open Respir Res (2021) 8(1):e000872. doi: 10.1136/bmjresp-2020-000872 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

