Ceftriaxone Suppresses Group II Metabotropic Glutamate Receptor Expression Contributing to Reversal of Recognition Memory Deficits of Amyloid Precursor Protein/Presenilin 1 AD Mice
- PMID: 35860293
- PMCID: PMC9289516
- DOI: 10.3389/fnins.2022.905403
Ceftriaxone Suppresses Group II Metabotropic Glutamate Receptor Expression Contributing to Reversal of Recognition Memory Deficits of Amyloid Precursor Protein/Presenilin 1 AD Mice
Abstract
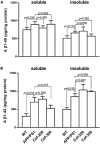
Group II metabotropic glutamate receptors (Group II mGluRs) are the peri-synaptic receptor of glutamatergic neurons and negatively regulate glutamate release from presynaptic neurons. Glutamate in the synaptic cleft is mainly taken into astrocytes by glutamate transporter-1 (GLT-1), which is primarily expressed in astrocytes. Increasing evidence showed that inhibiting or suppressing the activation of Group II mGluRs would contribute to the improvement of learning and memory deficits in Alzheimer's disease (AD) animal models. Ceftriaxone (Cef) has been reported to alleviate the spatial memory deficits in AD model mice by improving GLT-1-related clearance and metabolism of glutamate. Therefore, the present study further investigates the improving effect of Cef on recognition memory deficits and the involvement of Group II mGluRs in the process using the APP/PS1 AD mouse model. Novel object recognition tests showed that the Cef treatment significantly improved the recognition memory deficits of the AD mice. The Western blot and immunohistochemistry analysis showed that the Cef treatment significantly suppressed the upregulation of Group II mGluRs expression in APP/PS1 AD mice. The above suppression effect of Cef was blocked by dihydrokainic acid, an inhibitor of GLT-1 uptake activity. Furthermore, the Cef treatment significantly restored the downregulation in the downstream molecules of Group II mGluRs activation, including the expression of PKA and phosphorylated SNAP-25 in the APP/PS1 AD mice. The Cef treatment had no effect on the content of Aβ40 and Aβ42 in the hippocampus of APP/PS1 AD mice. The above results suggested that the suppression of Group II mGluRs contributed to the Cef-induced reversal of the recognition memory deficits in APP/PS1 AD mice.
Keywords: APP/PS1 mice; GLT-1; Group II mGluRs; SNAP-25; ceftriaxone.
Copyright © 2022 Fan, Li, Liu, Li, Xian and Li.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Ali I., Boets S., Janssens P., Van Eetveldt A., Amhaoul H., Langlois X., et al. (2016). Metabotropic glutamate receptor 2/3 density and its relation to the hippocampal neuropathology in a model of temporal lobe epilepsy in rats. Epilepsy Res. 127 55–59. 10.1016/j.eplepsyres.2016.08.010 - DOI - PubMed
-
- Anderson C. M., Swanson R. A. (2000). Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia 32 1–14. - PubMed
LinkOut - more resources
Full Text Sources

