A learning health system approach to the COVID-19 pandemic: System-wide changes in clinical practice and 30-day mortality among hospitalized patients
- PMID: 35860323
- PMCID: PMC9284933
- DOI: 10.1002/lrh2.10304
A learning health system approach to the COVID-19 pandemic: System-wide changes in clinical practice and 30-day mortality among hospitalized patients
Abstract
Introduction: Rapid, continuous implementation of credible scientific findings and regulatory approvals is often slow in large, diverse health systems. The coronavirus disease 2019 (COVID-19) pandemic created a new threat to this common "slow to learn and adapt" model in healthcare. We describe how the University of Pittsburgh Medical Center (UPMC) committed to a rapid learning health system (LHS) model to respond to the COVID-19 pandemic.
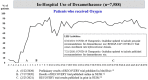
Methods: A treatment cohort study was conducted among 11 429 hospitalized patients (pediatric/adult) from 22 hospitals (PA, NY) with a primary diagnosis of COVID-19 infection (March 19, 2020 - June 6, 2021). Sociodemographic and clinical data were captured from UPMC electronic medical record (EMR) systems. Patients were grouped into four time-defined patient "waves" based on nadir of daily hospital admissions, with wave 3 (September 20, 2020 - March 10, 2021) split at its zenith due to high volume with steep acceleration and deceleration. Outcomes included changes in clinical practice (eg, use of corticosteroids, antivirals, and other therapies) in relation to timing of internal system analyses, scientific publications, and regulatory approvals, along with 30-day rate of mortality over time.
Results: The mean (SD) daily number of admissions across hospitals was 26 (29) with a maximum 7-day moving average of 107 patients. System-wide implementation of the use of dexamethasone, remdesivir, and tocilizumab occurred within days of release of corresponding seminal publications and regulatory actions. After adjustment for differences in patient clinical profiles over time, each month of hospital admission was associated with an estimated 5% lower odds of 30-day mortality (adjusted odds ratio [OR] = 0.95, 95% confidence interval: 0.93-0.97, P < .001).
Conclusions: In our large LHS, near real-time changes in clinical management of COVID-19 patients happened promptly as scientific publications and regulatory approvals occurred throughout the pandemic. Alongside these changes, patients with COVID-19 experienced lower adjusted 30-day mortality following hospital admission over time.
Keywords: dexamethasone; regulatory guidelines; remdesivir; scientific dissemination; temporal trends; tocilizumab.
© 2022 The Authors. Learning Health Systems published by Wiley Periodicals LLC on behalf of University of Michigan.
Conflict of interest statement
None of the authors received any payments or influence from a third‐party source for the work presented, and none report any potential conflicts of interest.
Figures


Similar articles
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Virtualized clinical studies to assess the natural history and impact of gut microbiome modulation in non-hospitalized patients with mild to moderate COVID-19 a randomized, open-label, prospective study with a parallel group study evaluating the physiologic effects of KB109 on gut microbiota structure and function: a structured summary of a study protocol for a randomized controlled study.Trials. 2021 Apr 2;22(1):245. doi: 10.1186/s13063-021-05157-0. Trials. 2021. PMID: 33810796 Free PMC article.
-
Investigational medications in 9,638 hospitalized patients with severe COVID-19: lessons from the "fail-and-learn" strategy during the first two waves of the pandemic in 2020.Patient Saf Surg. 2023 Apr 11;17(1):7. doi: 10.1186/s13037-023-00358-9. Patient Saf Surg. 2023. PMID: 37041643 Free PMC article.
-
Recombinant human C1 esterase inhibitor (conestat alfa) in the prevention of severe SARS-CoV-2 infection in hospitalized patients with COVID-19: A structured summary of a study protocol for a randomized, parallel-group, open-label, multi-center pilot trial (PROTECT-COVID-19).Trials. 2021 Jan 4;22(1):1. doi: 10.1186/s13063-020-04976-x. Trials. 2021. PMID: 33397449 Free PMC article.
-
Effects of short-term exposure to air pollution on hospital admissions of young children for acute lower respiratory infections in Ho Chi Minh City, Vietnam.Res Rep Health Eff Inst. 2012 Jun;(169):5-72; discussion 73-83. Res Rep Health Eff Inst. 2012. PMID: 22849236
Cited by
-
Clinical trials and their impact on policy during COVID-19: a review.Wellcome Open Res. 2024 Jan 30;9:20. doi: 10.12688/wellcomeopenres.19305.1. eCollection 2024. Wellcome Open Res. 2024. PMID: 38434720 Free PMC article. Review.
-
Evolving Real-World Effectiveness of Monoclonal Antibodies for Treatment of COVID-19 : A Cohort Study.Ann Intern Med. 2023 Apr;176(4):496-504. doi: 10.7326/M22-1286. Epub 2023 Apr 4. Ann Intern Med. 2023. PMID: 37011399 Free PMC article. Clinical Trial.
-
Designing and Implementing "Living and Breathing" Clinical Trials: An Overview and Lessons Learned from the COVID-19 Pandemic.Crit Care Clin. 2023 Oct;39(4):717-732. doi: 10.1016/j.ccc.2023.02.002. Epub 2023 Feb 17. Crit Care Clin. 2023. PMID: 37704336 Free PMC article. Review.
-
Progress with the Learning Health System 2.0: a rapid review of Learning Health Systems' responses to pandemics and climate change.BMC Med. 2024 Mar 22;22(1):131. doi: 10.1186/s12916-024-03345-8. BMC Med. 2024. PMID: 38519952 Free PMC article. Review.
-
Trajectories of Host-Response Subphenotypes in Patients With COVID-19 Across the Spectrum of Respiratory Support.CHEST Crit Care. 2023 Dec;1(3):100018. doi: 10.1016/j.chstcc.2023.100018. Epub 2023 Sep 14. CHEST Crit Care. 2023. PMID: 38250011 Free PMC article.
References
-
- Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID‐19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021. Feb;5(1):171‐176. doi:10.1016/j.mayocpiqo.2020.10.004 Epub 2020 Oct 29. PMID: 33163894; PMCID: PMC7598312. - DOI - PMC - PubMed
-
- Institute of Medicine (US) Roundtable on Evidence‐Based Medicine . The learning healthcare system. In: Olsen L, Aisner D, McGinnis JM, eds. Workshop Summary. Washington (DC): National Academies Press (US); 2007. PMID: 21452449. - PubMed
LinkOut - more resources
Full Text Sources

