Factors Affecting the Expression of Recombinant Protein and Improvement Strategies in Chinese Hamster Ovary Cells
- PMID: 35860329
- PMCID: PMC9289362
- DOI: 10.3389/fbioe.2022.880155
Factors Affecting the Expression of Recombinant Protein and Improvement Strategies in Chinese Hamster Ovary Cells
Abstract
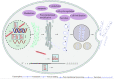
Recombinant therapeutic proteins (RTPs) are important parts of biopharmaceuticals. Chinese hamster ovary cells (CHO) have become the main cell hosts for the production of most RTPs approved for marketing because of their high-density suspension growth characteristics, and similar human post-translational modification patterns et al. In recent years, many studies have been performed on CHO cell expression systems, and the yields and quality of recombinant protein expression have been greatly improved. However, the expression levels of some proteins are still low or even difficult-to express in CHO cells. It is urgent further to increase the yields and to express successfully the "difficult-to express" protein in CHO cells. The process of recombinant protein expression of is a complex, involving multiple steps such as transcription, translation, folding processing and secretion. In addition, the inherent characteristics of molecular will also affect the production of protein. Here, we reviewed the factors affecting the expression of recombinant protein and improvement strategies in CHO cells.
Keywords: CHO; difficult to express; expression vector; recombinant protein; recombinant therapeutic proteins.
Copyright © 2022 Li, Fan, Wang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Factors and Mechanisms Affecting the Secretion of Recombinant Protein in CHO Cells.Curr Pharm Biotechnol. 2023;24(3):391-400. doi: 10.2174/1389201023666220603121316. Curr Pharm Biotechnol. 2023. PMID: 35658884 Review.
-
Effects and mechanism of small molecule additives on recombinant protein in CHO cells.Appl Microbiol Biotechnol. 2023 May;107(9):2771-2781. doi: 10.1007/s00253-023-12486-4. Epub 2023 Mar 27. Appl Microbiol Biotechnol. 2023. PMID: 36971794 Review.
-
Recombinant therapeutic proteins degradation and overcoming strategies in CHO cells.Appl Microbiol Biotechnol. 2024 Jan 29;108(1):182. doi: 10.1007/s00253-024-13008-6. Appl Microbiol Biotechnol. 2024. PMID: 38285115 Free PMC article. Review.
-
Effects and mechanisms of animal-free hydrolysates on recombination protein yields in CHO cells.Appl Microbiol Biotechnol. 2022 Nov;106(22):7387-7396. doi: 10.1007/s00253-022-12229-x. Epub 2022 Oct 14. Appl Microbiol Biotechnol. 2022. PMID: 36229612 Review.
-
Recombinant antibodies aggregation and overcoming strategies in CHO cells.Appl Microbiol Biotechnol. 2022 Jun;106(11):3913-3922. doi: 10.1007/s00253-022-11977-0. Epub 2022 May 24. Appl Microbiol Biotechnol. 2022. PMID: 35608667 Review.
Cited by
-
Time-Resolved Hierarchical Modeling Highlights Metabolites Influencing Productivity and Cell Death in Chinese Hamster Ovary Cells.Biotechnol J. 2025 Mar;20(3):e202400624. doi: 10.1002/biot.202400624. Biotechnol J. 2025. PMID: 40065671 Free PMC article.
-
Bioprocessing and the Production of Antiviral Biologics in the Prevention and Treatment of Viral Infectious Disease.Vaccines (Basel). 2023 May 17;11(5):992. doi: 10.3390/vaccines11050992. Vaccines (Basel). 2023. PMID: 37243096 Free PMC article. Review.
-
Construction of an Integration Vector with a Chimeric Signal Peptide for the Expression of Monoclonal Antibodies in Mammalian Cells.Curr Issues Mol Biol. 2024 Dec 22;46(12):14464-14475. doi: 10.3390/cimb46120868. Curr Issues Mol Biol. 2024. PMID: 39727996 Free PMC article.
-
Single-Cell Mitochondrial DNA Analysis of Recombinant Chinese Hamster Ovary Cells Reveals Widespread Heteroplasmy.Biotechnol J. 2025 Sep;20(9):e70079. doi: 10.1002/biot.70079. Biotechnol J. 2025. PMID: 40888103 Free PMC article.
-
Viral envelope proteins fused to multiple distinct fluorescent reporters to probe receptor binding.Protein Sci. 2024 Apr;33(4):e4974. doi: 10.1002/pro.4974. Protein Sci. 2024. PMID: 38533540 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

