Investigating discovery strategies and pharmacological properties of stereodefined phosphorodithioate LNA gapmers
- PMID: 35860384
- PMCID: PMC9271985
- DOI: 10.1016/j.omtn.2022.06.010
Investigating discovery strategies and pharmacological properties of stereodefined phosphorodithioate LNA gapmers
Abstract
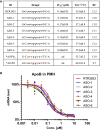
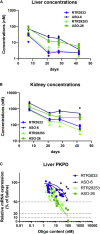
The introduction of sulfur into the phosphate linkage of chemically synthesized oligonucleotides creates the stereocenters on phosphorus atoms. Researchers have valued the nature of backbone stereochemistry and early on investigated drug properties for the individual stereocenters in dimers or short oligomers. Only very recently, it has become possible to synthesize fully stereodefined antisense oligonucleotides in good yield and purity. Non-bridging phosphorodithioate (PS2) introduces second sulfur into the phosphorothioate linkage to remove the chirality of phosphorus atom. Here, we describe the application of symmetrical non-bridging PS2 linkages in the context of stereodefined locked nucleic acids (LNAs) antisense oligonucleotides with the goal of reducing chiral complexity and, ultimately, resulting in single molecules. In addition, we propose a rather simple strategy to rapidly identify stereodefined gapmers, combining PS2 and a preferred stereochemistry motif (RSSR), which supports RNase-H-mediated target knockdown. Pharmacological efficacy and metabolic stability are investigated systematically using ApoB as a target sequence, where in vivo data correlate well to what is observed in vitro.
Keywords: MT: oligonucleotides: therapies and applications; antisense oligonucleotides; discovery strategies; locked nucleic acids; pharmacological properties; phosphorodithioates; stereodefined phosphorodithioates; symmetry.
© 2022 F.Hoffmann-La Roche AG.
Conflict of interest statement
J.D. is a member of a shareholder group with pooled voting rights of Roche as well as one of their representatives on the board of directors. J.W. is an academic collaborator. All the rest of the authors are or were employees of F. Hoffmann-La Roche AG.
Figures






Similar articles
-
The beauty of symmetry: siRNA phosphorodithioate modifications reduce stereocomplexity, ease analysis, and can improve in vivo potency.Mol Ther Nucleic Acids. 2024 Sep 10;35(4):102336. doi: 10.1016/j.omtn.2024.102336. eCollection 2024 Dec 10. Mol Ther Nucleic Acids. 2024. PMID: 39391764 Free PMC article.
-
Chemical Diversity of Locked Nucleic Acid-Modified Antisense Oligonucleotides Allows Optimization of Pharmaceutical Properties.Mol Ther Nucleic Acids. 2020 Mar 6;19:706-717. doi: 10.1016/j.omtn.2019.12.011. Epub 2019 Dec 18. Mol Ther Nucleic Acids. 2020. PMID: 31951854 Free PMC article.
-
An Investigation into the Potential of Targeting Escherichia coli rne mRNA with Locked Nucleic Acid (LNA) Gapmers as an Antibacterial Strategy.Molecules. 2021 Jun 4;26(11):3414. doi: 10.3390/molecules26113414. Molecules. 2021. PMID: 34200016 Free PMC article.
-
Locked nucleic acid oligonucleotides: the next generation of antisense agents?BioDrugs. 2007;21(4):235-43. doi: 10.2165/00063030-200721040-00004. BioDrugs. 2007. PMID: 17628121 Review.
-
Knocking Down Long Noncoding RNAs Using Antisense Oligonucleotide Gapmers.Methods Mol Biol. 2020;2176:49-56. doi: 10.1007/978-1-0716-0771-8_3. Methods Mol Biol. 2020. PMID: 32865781 Review.
Cited by
-
The beauty of symmetry: siRNA phosphorodithioate modifications reduce stereocomplexity, ease analysis, and can improve in vivo potency.Mol Ther Nucleic Acids. 2024 Sep 10;35(4):102336. doi: 10.1016/j.omtn.2024.102336. eCollection 2024 Dec 10. Mol Ther Nucleic Acids. 2024. PMID: 39391764 Free PMC article.
-
Discovery and characterization of stereodefined PMO-gapmers targeting tau.Mol Ther Nucleic Acids. 2024 Nov 28;36(1):102404. doi: 10.1016/j.omtn.2024.102404. eCollection 2025 Mar 11. Mol Ther Nucleic Acids. 2024. PMID: 39759873 Free PMC article.
References
-
- Hagedorn P.H., Persson R., Funder E.D., Albaek N., Diemer S.L., Hansen D.J., Moller M.R., Papargyri N., Christiansen H., Hansen B.R., et al. Locked nucleic acid: modality, diversity, and drug discovery. Drug Discov. Today. 2018;23:101–114. - PubMed
-
- Guga P., Stec W.J. Synthesis of phosphorothioate oligonucleotides with stereodefined phosphorothioate linkages. Curr. Protoc. Nucleic Acid Chem. 2003;Chapter 4:Unit 4.17. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

