Conservation of miR combo based direct cardiac reprogramming
- PMID: 35860436
- PMCID: PMC9293594
- DOI: 10.1016/j.bbrep.2022.101310
Conservation of miR combo based direct cardiac reprogramming
Abstract
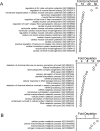
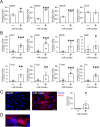
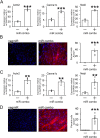
There is considerable interest in regenerating the injured heart by reprogramming resident fibroblasts into new functional cardiomyocytes. Cardiac reprogramming has been achieved via transcription factors or miRNAs. Transcription factor combinations appear to be species-specific as evidenced by the fact that combinations of transcription factors which are effective for the reprogramming of mouse fibroblasts are ineffective in pigs and humans. Whether miRNA based cardiac reprogramming suffers from the same limitation is unknown. We have previously demonstrated that mouse cardiac fibroblasts can be directly converted into cardiomyocytes both in vitro and in vivo via a combination of four microRNAs (miR-1, miR-133a, miR-208a and miR-499) termed "miR combo." To assess species-specificity, miR combo was transfected into cardiac fibroblasts isolated from the left ventricle of dogs, pigs and humans. QPCR analysis indicated that miR combo effectively reprogrammed fibroblasts from all of the tested mammalian species. Significant upregulation of cardiac developmental, sarcomere, and cardiac ion channel genes was observed. Through Actn2+ staining, we also found that miR combo transfection induced dog, pig and human cardiac fibroblasts to develop into cardiomyocyte-like cells. In conclusion, we have demonstrated that in contrast to transcription factor based approaches, miR combo effectively reprograms mammalian cardiac fibroblasts into cardiomyocyte-like cells.
Keywords: Cardiomyocytes; Fibroblasts; Reprogramming; miRNAs.
© 2022 The Authors.
Conflict of interest statement
Conrad Hodgkinson is a co-founder of Recardia Therapeutics. This company is focused on developing miRNAs that reprogram fibroblasts into cardiomyocytes.
Figures
Similar articles
-
Demethylation of H3K27 Is Essential for the Induction of Direct Cardiac Reprogramming by miR Combo.Circ Res. 2017 Apr 28;120(9):1403-1413. doi: 10.1161/CIRCRESAHA.116.308741. Epub 2017 Feb 16. Circ Res. 2017. PMID: 28209718 Free PMC article.
-
Optimizing delivery for efficient cardiac reprogramming.Biochem Biophys Res Commun. 2020 Nov 26;533(1):9-16. doi: 10.1016/j.bbrc.2020.08.104. Epub 2020 Sep 9. Biochem Biophys Res Commun. 2020. PMID: 32917363 Free PMC article.
-
Production of Cardiomyocytes by microRNA-Mediated Reprogramming in Optimized Reprogramming Media.Methods Mol Biol. 2021;2239:47-59. doi: 10.1007/978-1-0716-1084-8_4. Methods Mol Biol. 2021. PMID: 33226612
-
Reprogramming of Non-myocytes into Cardiomyocyte-like Cells: Challenges and Opportunities.Curr Cardiol Rep. 2020 Jun 19;22(8):54. doi: 10.1007/s11886-020-01322-0. Curr Cardiol Rep. 2020. PMID: 32562156 Review.
-
MiRroring the Multiple Potentials of MicroRNAs in Acute Myocardial Infarction.Front Cardiovasc Med. 2017 Nov 20;4:73. doi: 10.3389/fcvm.2017.00073. eCollection 2017. Front Cardiovasc Med. 2017. PMID: 29209617 Free PMC article. Review.
Cited by
-
The Potential of RNA Therapeutics in Treating Cardiovascular Disease.Drugs. 2025 May;85(5):659-676. doi: 10.1007/s40265-025-02173-1. Epub 2025 Apr 2. Drugs. 2025. PMID: 40175855 Review.
-
RNA Therapies in Cardio-Kidney-Metabolic Syndrome: Advancing Disease Management.J Cardiovasc Transl Res. 2025 Mar 13. doi: 10.1007/s12265-025-10603-4. Online ahead of print. J Cardiovasc Transl Res. 2025. PMID: 40080261 Review.
-
Modifying miRs for effective reprogramming of fibroblasts to cardiomyocytes.Mol Ther Nucleic Acids. 2024 Feb 28;35(2):102160. doi: 10.1016/j.omtn.2024.102160. eCollection 2024 Jun 11. Mol Ther Nucleic Acids. 2024. PMID: 38495845 Free PMC article.
-
C166 EVs potentiate miR cardiac reprogramming via miR-148a-3p.J Mol Cell Cardiol. 2024 May;190:48-61. doi: 10.1016/j.yjmcc.2024.04.002. Epub 2024 Apr 4. J Mol Cell Cardiol. 2024. PMID: 38582260 Free PMC article.
-
Cell reprogramming: methods, mechanisms and applications.Cell Regen. 2025 Mar 27;14(1):12. doi: 10.1186/s13619-025-00229-x. Cell Regen. 2025. PMID: 40140235 Free PMC article. Review.
References
-
- Vp Singh M.M., Xu X., Patel V.K., Belaguli N.S., Gibson B.W., Cooney A.J., Rosengart T.K. Reprogramming of pig cardiac fibroblasts to cardiomyocyte fate: implications for gene therapy to treat myocardial infarction. Circ. Res. 2014;115
LinkOut - more resources
Full Text Sources

