Emerging High-Frequency Ultrasound Imaging in Medical Cosmetology
- PMID: 35860664
- PMCID: PMC9289277
- DOI: 10.3389/fphys.2022.885922
Emerging High-Frequency Ultrasound Imaging in Medical Cosmetology
Abstract
Cosmetic skin diseases are a part of many dermatological concerns brought up by patients, which negatively affect mental health and quality of life. Imaging technology has an established role in the diagnosis of cosmetic skin diseases by recognizing information on deep skin lesions. Due to the complex physiological and pathological nature of cosmetic skin diseases, the diagnostic imaging performance varies greatly. Developing noninvasive technology models with wide applicability, particularly high-frequency ultrasound (HFUS), which is able to achieve high-resolution imaging of the skin from the stratum corneum down to the deep fascia, is of great significance to medical cosmetology. To explore the great potential of HFUS in cosmetic skin diseases, a narrative review of literature from PubMed and Web of Science published between 1985 and 2022 was conducted. This narrative review focuses on the progression of HFUS imaging in medical cosmetology, especially on its promising application in the quantitative evaluation and differential diagnosis of cutaneous pathological scar, port wine stain (PWS), acne, skin aging, and other cosmetic applications.
Keywords: dermatology; high-frequency ultrasound; medical cosmetology; pathological scar; port wine stain.
Copyright © 2022 Tao, Wei, Su, Hu and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
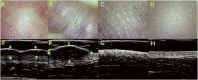
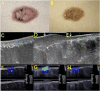
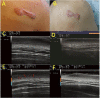
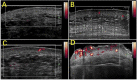
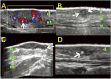
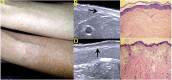
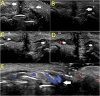
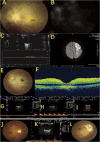
Similar articles
-
High-frequency ultrasound as a scientific tool for skin imaging analysis.Exp Dermatol. 2021 Jul;30(7):897-910. doi: 10.1111/exd.14363. Epub 2021 May 7. Exp Dermatol. 2021. PMID: 33905589 Review.
-
High-frequency ultrasound in clinical dermatology: a review.Ultrasound J. 2021 Apr 20;13(1):24. doi: 10.1186/s13089-021-00222-w. Ultrasound J. 2021. PMID: 33877462 Free PMC article. Review.
-
Application of different noninvasive diagnostic techniques used in HMME-PDT in the treatment of port wine stains.Photodiagnosis Photodyn Ther. 2019 Mar;25:369-375. doi: 10.1016/j.pdpdt.2019.01.008. Epub 2019 Jan 6. Photodiagnosis Photodyn Ther. 2019. PMID: 30625397
-
Noninvasive diagnostic techniques of port wine stain.J Cosmet Dermatol. 2021 Jul;20(7):2006-2014. doi: 10.1111/jocd.14087. Epub 2021 Mar 31. J Cosmet Dermatol. 2021. PMID: 33788368 Review.
-
THE IMPORTANCE OF REMOTE COUNSELING IN COSMETOLOGY AND COSMETIC DERMATOLOGY.Pol Merkur Lekarski. 2023;51(1):74-87. doi: 10.36740/Merkur202301111. Pol Merkur Lekarski. 2023. PMID: 36960904 Review.
Cited by
-
Objective Noninvasive Measurement of the Volumizing Effect of a Dermal Filler: An In Vivo Study.Aesthetic Plast Surg. 2024 Oct;48(19):4024-4030. doi: 10.1007/s00266-024-04138-3. Epub 2024 May 28. Aesthetic Plast Surg. 2024. PMID: 38806832 Free PMC article.
-
Evaluating Effects of Skin Needling Treatment on Visible Changes and Elasticity of Scars Using High-Frequency Ultrasound, Cutometer®, and Standardized Questionnaire-Six Case Studies.J Clin Med. 2025 Aug 6;14(15):5553. doi: 10.3390/jcm14155553. J Clin Med. 2025. PMID: 40807173 Free PMC article.
-
Advances in the Application of Noninvasive Skin Imaging Techniques in Acne Scars.Am J Clin Dermatol. 2024 Sep;25(5):823-835. doi: 10.1007/s40257-024-00882-z. Epub 2024 Aug 12. Am J Clin Dermatol. 2024. PMID: 39134786 Review.
-
The application of tissue engineering strategies for uterine regeneration.Mater Today Bio. 2025 Feb 18;31:101594. doi: 10.1016/j.mtbio.2025.101594. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40070871 Free PMC article. Review.
-
High-Frequency and Ultra-High-Frequency Ultrasound in Dermatologic Diseases and Aesthetic Medicine.Medicina (Kaunas). 2025 Jan 26;61(2):220. doi: 10.3390/medicina61020220. Medicina (Kaunas). 2025. PMID: 40005337 Free PMC article. Review.
References
-
- Ardigo M., Cameli N., Berardesca E., Gonzalez S. (2010). Characterization and Evaluation of Pigment Distribution and Response to Therapy in Melasma Using In Vivo Reflectance Confocal Microscopy: a Preliminary Study. J. Eur. Acad. Dermatol Venereol. 24 (11), 1296–1303. 10.1111/j.1468-3083.2010.03633.x - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

