Guidelines for COVID-19 Laboratory Testing for Emergency Departments From the New Diagnostic Technology Team of the Taiwan Society of Emergency Medicine
- PMID: 35860709
- PMCID: PMC9283118
- DOI: 10.6705/j.jacme.202206_12(2).0001
Guidelines for COVID-19 Laboratory Testing for Emergency Departments From the New Diagnostic Technology Team of the Taiwan Society of Emergency Medicine
Abstract
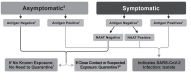
COVID-19 tests have different turnaround times (TATs), accuracy levels, and limitations, which emergency physicians should be aware of. Nucleic acid amplification tests (NAATs) can be divided into standard high throughput tests and rapid molecular diagnostic tests at the point of care (POC). The standard NAAT has the advantages of high throughput and high accuracy with a TAT of 3-4 hours. The POC molecular test has the same advantages of high accuracy as standard high throughput PCR, but can be done in 13-45 minutes. Roche cobas Liat is the most commonly used machine in Taiwan, displaying 99%-100% sensitivity and 100% specificity, respectively. Abbott ID NOW is an isothermal PCR-based POC machine with a sensitivity of 79% and a specificity of 100%. A high rate of false positives and false negatives is associated with rapid antigen testing. Antibody testing is mostly used as part of public health surveys and for testing for immunity.
Keywords: COVID-19; nucleic acid amplification testing; point-of-care testing; rapid point-of-care molecular testing.
Figures
Similar articles
-
Clinical Performance of the Point-of-Care cobas Liat for Detection of SARS-CoV-2 in 20 Minutes: a Multicenter Study.J Clin Microbiol. 2021 Jan 21;59(2):e02811-20. doi: 10.1128/JCM.02811-20. Print 2021 Jan 21. J Clin Microbiol. 2021. PMID: 33239382 Free PMC article.
-
Sensitivity and potential utility of SARS-CoV-2 rapid antigen and nucleic acid amplification tests in the context of an elimination approach.N Z Med J. 2021 Nov 26;134(1546):28-37. N Z Med J. 2021. PMID: 34855731
-
Effectiveness and use of reverse transcriptase polymerase chain reaction point of care testing in a large-scale COVID-19 surveillance system.Pharmacoepidemiol Drug Saf. 2022 May;31(5):511-518. doi: 10.1002/pds.5424. Epub 2022 Mar 11. Pharmacoepidemiol Drug Saf. 2022. PMID: 35225407 Free PMC article.
-
The performance of non-NAAT point-of-care (POC) tests and rapid NAAT tests for chlamydia and gonorrhoea infections. An assessment of currently available assays.Sex Transm Infect. 2015 Dec;91(8):539-44. doi: 10.1136/sextrans-2014-051997. Epub 2015 May 2. Sex Transm Infect. 2015. PMID: 25935930 Review.
-
Advances in nucleic acid amplification techniques (NAATs): COVID-19 point-of-care diagnostics as an example.Biosens Bioelectron. 2022 Jun 15;206:114109. doi: 10.1016/j.bios.2022.114109. Epub 2022 Feb 26. Biosens Bioelectron. 2022. PMID: 35245867 Review.
Cited by
-
Breaking Boundaries in Pneumonia Diagnostics: Transitioning from Tradition to Molecular Frontiers with Multiplex PCR.Diagnostics (Basel). 2024 Apr 2;14(7):752. doi: 10.3390/diagnostics14070752. Diagnostics (Basel). 2024. PMID: 38611665 Free PMC article. Review.
-
[Implementation of isotermic PCR for detection of SARS-CoV-2 virus].Rev Med Inst Mex Seguro Soc. 2024 May 6;62(3):1-6. doi: 10.5281/zenodo.10998931. Rev Med Inst Mex Seguro Soc. 2024. PMID: 39530769 Free PMC article. Spanish.
-
Prevalence and healthcare burden of inappropriate antimicrobial treatment in patients at high risk of complications from acute respiratory infections: a scoping review.Front Med (Lausanne). 2025 May 16;12:1533797. doi: 10.3389/fmed.2025.1533797. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40454140 Free PMC article.
References
-
- Guidance for antigen testing for SARS-CoV-2 for healthcare providers testing individuals in the community. Centers for Disease Control and Prevention. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-gu.... Published September 9, 2021. Accessed October 22, 2021.
LinkOut - more resources
Full Text Sources
