Benign inheritable disorders of bilirubin metabolism manifested by conjugated hyperbilirubinemia-A narrative review
- PMID: 35860851
- PMCID: PMC9486497
- DOI: 10.1002/ueg2.12279
Benign inheritable disorders of bilirubin metabolism manifested by conjugated hyperbilirubinemia-A narrative review
Abstract
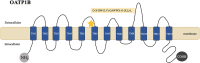
Bilirubin, a breakdown product of heme, is normally glucuronidated and excreted by the liver into bile. Failure of this system can lead to a buildup of conjugated bilirubin in the blood, resulting in jaundice. Hyperbilirubinemia is an important clinical sign that needs to be investigated under a stepwise evaluation. Inherited non-hemolytic conjugated hyperbilirubinemic conditions include Dubin-Johnson syndrome (caused by mutations affecting ABCC2 gene) and Rotor syndrome (caused by the simultaneous presence of mutations in SLCO1B1 and SLCO1B3 genes). Although classically viewed as benign conditions requiring no treatment, they lately gained an increased interest since recent studies suggested that mutations in the responsible genes leading to hyperbilirubinemia, as well as minor genetic variants, may result in an increased susceptibility to drug toxicity. This article provides a comprehensive review on the pathophysiology of Dubin-Johnson and Rotor syndromes, presenting the current knowledge concerning the molecular details and basis of these conditions.
Keywords: ABCC2/MRP2; Dubin-Johnson syndrome; Rotor syndrome; SLCO1B1/OATP1B1; SLCO1B3/OATP1B3; conjugated hyperbilirubinemia.
© 2022 The Authors. United European Gastroenterology Journal published by Wiley Periodicals LLC on behalf of United European Gastroenterology.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

