Early evaluation of a next-generation surgical system in robot-assisted total laparoscopic hysterectomy: A prospective clinical cohort study
- PMID: 35861102
- PMCID: PMC9564672
- DOI: 10.1111/aogs.14407
Early evaluation of a next-generation surgical system in robot-assisted total laparoscopic hysterectomy: A prospective clinical cohort study
Abstract
Introduction: This study aimed to demonstrate the safe and effective use of the Versius surgical system (CMR Surgical, Cambridge, UK) in robot-assisted total laparoscopic hysterectomy. This surgical robot was developed iteratively with input from surgeons to improve surgical outcomes and end-user experience. We report data from the gynecology cohort of an early clinical trial designed in broad alignment with IDEAL-D (Idea, Development, Exploration, Assessment, Long-term follow-up - Devices) stage 2b (Exploration).
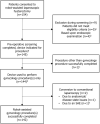
Material and methods: The study is registered in the Indian clinical trials register (CTRI/2019/02/017872). Adult women requiring total hysterectomy who provided informed consent and met the eligibility criteria underwent procedures at one of three hospitals in India. Five surgeons performed robot-assisted total laparoscopic hysterectomies using the device from March 2019 to September 2020. The primary endpoint was rate of unplanned conversion to conventional laparoscopic or open surgery. Adverse events were adjudicated by an independent clinical events committee using endoscope video recordings and clinical notes.
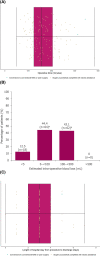
Results: In total, 144 women underwent surgery (median age: 44 years [range: 28-78]; median body mass index 25.8 kg/m2 [range: 14.3-47.8]). The rate of unplanned conversion to conventional laparoscopy was 2/144 (1.4%); neither conversion was device related. No surgery was converted to open. In total, 13 adverse events occurred among seven (4.9%) patients, comprising seven serious adverse events and six adverse events. One serious adverse event was deemed device-related. Two patients were readmitted to hospital within 30 days; both made a full recovery. No patients died within 90 days of surgery.
Conclusions: The device provides a safe and effective option for total laparoscopic hysterectomy; these findings support its continued implementation in larger patient cohorts and expansion in other major minimal access indications.
Keywords: clinical trial; gynecology; minimal access surgery; robot-assisted surgery; robotic surgical system; surgical robot.
© 2022 The Authors. Acta Obstetricia et Gynecologica Scandinavica published by John Wiley & Sons Ltd on behalf of Nordic Federation of Societies of Obstetrics and Gynecology (NFOG).
Conflict of interest statement
ED is a paid consultant for CMR Surgical, and MS is Chief Medical Officer and founder of CMR Surgical. The remaining authors have no conflicts of interest to declare.
Figures

References
-
- He H, Zeng D, Ou H, Tang YZ, Li JJ, Zhong H. Laparoscopic treatment of endometrial cancer: systematic review. J Minim Invasive Gynecol. 2013;20:413‐423. - PubMed
-
- Xu T, Hutfless SM, Cooper MA, Zhou M, Massie AB, Makary MA. Hospital cost implications of increased use of minimally invasive surgery. JAMA Surg. 2015;150:489‐490. - PubMed
-
- Garcia‐Ruiz A, Gagner M, Miller JH, Steiner CP, Hahn JF. Manual vs robotically assisted laparoscopic surgery in the performance of basic manipulation and suturing tasks. Archives Surg. 1998;133:957‐961. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

