A randomized controlled trial to evaluate efficacy and safety of early conversion to a low-dose calcineurin inhibitor combined with sirolimus in renal transplant patients
- PMID: 35861301
- PMCID: PMC9532037
- DOI: 10.1097/CM9.0000000000001866
A randomized controlled trial to evaluate efficacy and safety of early conversion to a low-dose calcineurin inhibitor combined with sirolimus in renal transplant patients
Abstract
Background: The calcineurin inhibitor (CNI)-based immune maintenance regimen that is commonly used after renal transplantation has greatly improved early graft survival after transplantation; however, the long-term prognosis of grafts has not been significantly improved. The nephrotoxicity of CNI drugs is one of the main risk factors for the poor long-term prognosis of grafts. Sirolimus (SRL) has been employed as an immunosuppressant in clinical practice for over 20 years and has been found to have no nephrotoxic effects on grafts. Presently, the regimen and timing of SRL application after renal transplantation vary, and clinical data are scarce. Multicenter prospective randomized controlled studies are particularly rare. This study aims to investigate the effects of early conversion to a low-dose CNI combined with SRL on the long-term prognosis of renal transplantation.
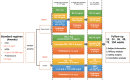
Methods: Patients who receive four weeks of a standard regimen with CNI + mycophenolic acid (MPA) + glucocorticoid after renal transplantation in multiple transplant centers across China will be included in this study. At week 5, after the operation, patients in the experimental group will receive an additional administration of SRL, a reduction in the CNI drug doses, withdrawal of MPA medication, and maintenance of glucocorticoids. In addition, patients in the control group will receive the maintained standard of care. The patients' vital signs, routine blood tests, routine urine tests, blood biochemistry, serum creatinine, BK virus (BKV)/ cytomegalovirus (CMV), and trough concentrations of CNI drugs and SRL at the baseline and weeks 12, 24, 36, 48, 72, and 104 after conversion will be recorded. Patient survival, graft survival, and estimated glomerular filtration rate will be calculated, and concomitant medications and adverse events will also be recorded.
Conclusion: The study data will be utilized to evaluate the efficacy and safety of early conversion to low-dose CNIs combined with SRL in renal transplant patients.
Trial registration: Chinese Clinical Trial Registry, ChiCTR1800017277.
Copyright © 2022 The Chinese Medical Association, produced by Wolters Kluwer, Inc. under the CC-BY-NC-ND license.
Conflict of interest statement
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled.
Figures
References
-
- Overbeck I, Bartels M, Decker O, Harms J, Hauss J, Fangmann J. Changes in quality of life after renal transplantation. Transplant Proc 2005; 37:1618–1621. doi: 10.1016/j.transproceed.2004.09.019. - PubMed
-
- Schold JD, Meier-Kriesche HU. Which renal transplant candidates should accept marginal kidneys in exchange for a shorter waiting time on dialysis? Clin J Am Soc Nephrol 2006; 1:532–538. doi: 10.2215/cjn.01130905. - PubMed
-
- Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, et al. United states renal data system 2011 annual data report: atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis 2012; 59: (suppl 1 A7): e1–e420. doi: 10.1053/j.ajkd.2011.11.015. - PubMed
-
- Annual Data Report of the US Organ Procurement and Transplantation Network (OPTN) and the Scientific Registry of Transplant Recipients (SRTR). Preface. Am J Transplant 2013; 13: (suppl 1): 1–7. doi: 10.1111/ajt.12028. - PubMed
-
- Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant 2004; 4:378–383. doi: 10.1111/j.1600-6143.2004.00332.x. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous