Mapping odorant sensitivities reveals a sparse but structured representation of olfactory chemical space by sensory input to the mouse olfactory bulb
- PMID: 35861321
- PMCID: PMC9352350
- DOI: 10.7554/eLife.80470
Mapping odorant sensitivities reveals a sparse but structured representation of olfactory chemical space by sensory input to the mouse olfactory bulb
Abstract
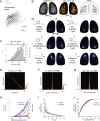
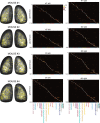
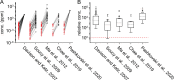
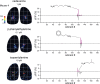
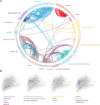
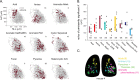
In olfactory systems, convergence of sensory neurons onto glomeruli generates a map of odorant receptor identity. How glomerular maps relate to sensory space remains unclear. We sought to better characterize this relationship in the mouse olfactory system by defining glomeruli in terms of the odorants to which they are most sensitive. Using high-throughput odorant delivery and ultrasensitive imaging of sensory inputs, we imaged responses to 185 odorants presented at concentrations determined to activate only one or a few glomeruli across the dorsal olfactory bulb. The resulting datasets defined the tuning properties of glomeruli - and, by inference, their cognate odorant receptors - in a low-concentration regime, and yielded consensus maps of glomerular sensitivity across a wide range of chemical space. Glomeruli were extremely narrowly tuned, with ~25% responding to only one odorant, and extremely sensitive, responding to their effective odorants at sub-picomolar to nanomolar concentrations. Such narrow tuning in this concentration regime allowed for reliable functional identification of many glomeruli based on a single diagnostic odorant. At the same time, the response spectra of glomeruli responding to multiple odorants was best predicted by straightforward odorant structural features, and glomeruli sensitive to distinct odorants with common structural features were spatially clustered. These results define an underlying structure to the primary representation of sensory space by the mouse olfactory system.
Keywords: chemoinformatics; coding; imaging; mouse; neuroscience; odor; olfactometry.
© 2022, Burton et al.
Conflict of interest statement
SB, AB, TE, IY, TR, MS, MW No competing interests declared
Figures








Similar articles
-
Recovery of glomerular morphology in the olfactory bulb of young mice after disruption caused by continuous odorant exposure.Brain Res. 2017 Sep 1;1670:6-13. doi: 10.1016/j.brainres.2017.05.030. Epub 2017 Jun 2. Brain Res. 2017. PMID: 28583862
-
Representation of odorants by receptor neuron input to the mouse olfactory bulb.Neuron. 2001 Nov 20;32(4):723-35. doi: 10.1016/s0896-6273(01)00506-2. Neuron. 2001. PMID: 11719211
-
Grouping and representation of odorant receptors in domains of the olfactory bulb sensory map.Microsc Res Tech. 2002 Aug 1;58(3):168-75. doi: 10.1002/jemt.10146. Microsc Res Tech. 2002. PMID: 12203695 Review.
-
Interglomerular center-surround inhibition shapes odorant-evoked input to the mouse olfactory bulb in vivo.J Neurophysiol. 2006 Mar;95(3):1881-7. doi: 10.1152/jn.00918.2005. Epub 2005 Nov 30. J Neurophysiol. 2006. PMID: 16319205
-
The olfactory bulb: coding and processing of odor molecule information.Science. 1999 Oct 22;286(5440):711-5. doi: 10.1126/science.286.5440.711. Science. 1999. PMID: 10531048 Review.
Cited by
-
More than meets the AI: The possibilities and limits of machine learning in olfaction.Front Neurosci. 2022 Sep 1;16:981294. doi: 10.3389/fnins.2022.981294. eCollection 2022. Front Neurosci. 2022. PMID: 36117640 Free PMC article. Review.
-
Robust odor identification in novel olfactory environments in mice.Nat Commun. 2023 Feb 13;14(1):673. doi: 10.1038/s41467-023-36346-x. Nat Commun. 2023. PMID: 36781878 Free PMC article.
-
Recalibrating Olfactory Neuroscience to the Range of Naturally Occurring Odor Concentrations.J Neurosci. 2025 Mar 5;45(10):e1872242024. doi: 10.1523/JNEUROSCI.1872-24.2024. J Neurosci. 2025. PMID: 40044450 Review.
-
The behavioral sensitivity of mice to acyclic, monocyclic, and bicyclic monoterpenes.PLoS One. 2024 Feb 23;19(2):e0298448. doi: 10.1371/journal.pone.0298448. eCollection 2024. PLoS One. 2024. PMID: 38394306 Free PMC article.
-
Anterior Olfactory Cortices Differentially Transform Bottom-Up Odor Signals to Produce Inverse Top-Down Outputs.J Neurosci. 2024 Oct 30;44(44):e0231242024. doi: 10.1523/JNEUROSCI.0231-24.2024. J Neurosci. 2024. PMID: 39266300 Free PMC article.
References
-
- Bach A, Yáñez-Serrano AM, Llusià J, Filella I, Maneja R, Penuelas J. Human breathable air in a mediterranean forest: Characterization of monoterpene concentrations under the canopy. International Journal of Environmental Research and Public Health. 2020;17:4391. doi: 10.3390/ijerph17124391. - DOI - PMC - PubMed
-
- Bozza T, Vassalli A, Fuss S, Zhang JJ, Weiland B, Pacifico R, Feinstein P, Mombaerts P. Mapping of class I and class II odorant receptors to glomerular domains by two distinct types of olfactory sensory neurons in the mouse. Neuron. 2009;61:220–233. doi: 10.1016/j.neuron.2008.11.010. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

