Highly Aligned Bacterial Nanocellulose Films Obtained During Static Biosynthesis in a Reproducible and Straightforward Approach
- PMID: 35861401
- PMCID: PMC9475533
- DOI: 10.1002/advs.202201947
Highly Aligned Bacterial Nanocellulose Films Obtained During Static Biosynthesis in a Reproducible and Straightforward Approach
Abstract
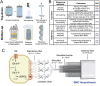
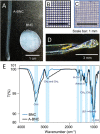
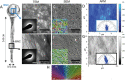
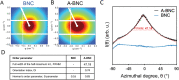
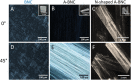
Bacterial nanocellulose (BNC) is usually produced as randomly-organized highly pure cellulose nanofibers films. Its high water-holding capacity, porosity, mechanical strength, and biocompatibility make it unique. Ordered structures are found in nature and the properties appearing upon aligning polymers fibers inspire everyone to achieve highly aligned BNC (A-BNC) films. This work takes advantage of natural bacteria biosynthesis in a reproducible and straightforward approach. Bacteria confined and statically incubated biosynthesized BNC nanofibers in a single direction without entanglement. The obtained film is highly oriented within the total volume confirmed by polarization-resolved second-harmonic generation signal and Small Angle X-ray Scattering. The biosynthesis approach is improved by reusing the bacterial substrates to obtain A-BNC reproducibly and repeatedly. The suitability of A-BNC as cell carriers is confirmed by adhering to and growing fibroblasts in the substrate. Finally, the thermal conductivity is evaluated by two independent approaches, i.e., using the well-known 3ω-method and a recently developed contactless thermoreflectance approach, confirming a thermal conductivity of 1.63 W mK-1 in the direction of the aligned fibers versus 0.3 W mK-1 perpendicularly. The fivefold increase in thermal conductivity of BNC in the alignment direction forecasts the potential of BNC-based devices outperforming some other natural polymer and synthetic materials.
Keywords: aligned fibers; anisotropy; bacterial nanocellulose; biofabrication; bioinspiration; hydrogels; nanomaterials; sustainability; thermal conductivity.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Wan J., Song J., Yang Z., Kirsch D., Jia C., Xu R., Dai J., Zhu M., Xu L., Chen C., Wang Y., Wang Y., Hitz E., Lacey S. D., Li Y., Yang B., Hu L., Adv. Mater. 2017, 29, 1703331. - PubMed
MeSH terms
Substances
Grants and funding
- PID2019-104228RB-I00/MCIN/AEI//10.13039/501100011033/FEDER "Una manera de hacer Europa,"
- 2017SGR765/MCIN/AEI/10.13039/501100011033, the Generalitat de Catalunya
- SEV-2015-0496/"Severo Ochoa" Programme for Centres of Excellence in R&D
- CEX2019-000917-S/"Severo Ochoa" Programme for Centres of Excellence in R&D
- PID2020-119777GB-I00/FUNFUTURE
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
