Treatable traits in the NOVELTY study
- PMID: 35861464
- PMCID: PMC9795904
- DOI: 10.1111/resp.14325
Treatable traits in the NOVELTY study
Erratum in
-
Corrigendum.Respirology. 2022 Dec;27(12):1095. doi: 10.1111/resp.14406. Epub 2022 Nov 6. Respirology. 2022. PMID: 36336467 Free PMC article. No abstract available.
Abstract
Background and objective: Asthma and chronic obstructive pulmonary disease (COPD) are two prevalent and complex diseases that require personalized management. Although a strategy based on treatable traits (TTs) has been proposed, the prevalence and relationship of TTs to the diagnostic label and disease severity established by the attending physician in a real-world setting are unknown. We assessed how the presence/absence of specific TTs relate to the diagnosis and severity of 'asthma', 'COPD' or 'asthma + COPD'.
Methods: The authors selected 30 frequently occurring TTs from the NOVELTY study cohort (NOVEL observational longiTudinal studY; NCT02760329), a large (n = 11,226), global study that systematically collects data in a real-world setting, both in primary care clinics and specialized centres, for patients with 'asthma' (n = 5932, 52.8%), 'COPD' (n = 3898, 34.7%) or both ('asthma + COPD'; n = 1396, 12.4%).
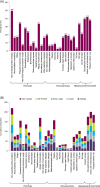
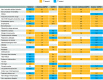
Results: The results indicate that (1) the prevalence of the 30 TTs evaluated varied widely, with a mean ± SD of 4.6 ± 2.6, 5.4 ± 2.6 and 6.4 ± 2.8 TTs/patient in those with 'asthma', 'COPD' and 'asthma + COPD', respectively (p < 0.0001); (2) there were no large global geographical variations, but the prevalence of TTs was different in primary versus specialized clinics; (3) several TTs were specific to the diagnosis and severity of disease, but many were not; and (4) both the presence and absence of TTs formed a pattern that is recognized by clinicians to establish a diagnosis and grade its severity.
Conclusion: These results provide the largest and most granular characterization of TTs in patients with airway diseases in a real-world setting to date.
Keywords: COPD; airways; allergy; asthma; bronchitis; chronic obstructive pulmonary disease; emphysema; smoking.
© 2022 The Authors. Respirology published by John Wiley & Sons Australia, Ltd on behalf of Asian Pacific Society of Respirology.
Conflict of interest statement
Alvar Agustí has received payments in his role as a member of the scientific committee of NOVELTY; received research grants from AstraZeneca, Chiesi, GSK, Menarini, MSD and Zambon; consulting fees from AstraZeneca, Chiesi, GSK, Menarini, MSD and Zambon; and payment or honoraria from AstraZeneca, Chiesi, GSK, Menarini, MSD and Zambon. Eleni Rapsomaniki and Rod Hughes are employees of AstraZeneca. Hana Müllerová is an employee and shareholder of AstraZeneca. Richard Beasley received research grants from AstraZeneca, Cure Kids (NZ), Genentech, GSK and HRC (NZ); consulting fees from AstraZeneca, Avillion and Theravance; payment or honoraria from AstraZeneca, Cipla and Asthma and Respiratory Foundation NZ; support for attending meetings from AstraZeneca and Theravance; and is the Chair of the adult asthma guidelines group for the Asthma and Respiratory Foundation of NZ. Alberto Papi received research grants from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Pfizer, Sanofi and Teva; consulting fees from AstraZeneca, Avillion, Chiesi, Elpen Pharmaceuticals, GSK, IQVIA, Novartis and Sanofi; and payment or honoraria from AstraZeneca, Avillion, Boehringer Ingelheim, Chiesi, Edmond Pharma, Elpen Pharmaceuticals, GSK, IQVIA, Menarini, MSD, Mundipharma, Novartis, Sanofi, Teva and Zambon. Ian D. Pavord received research grants from Chiesi; consulting fees from Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Circassia, Dey Pharma, Genentech, GSK, Knopp Biosciences, Merck, MSD, Napp Pharmaceuticals, Novartis, Regeneron Pharmaceuticals Inc., Respivert, Schering‐Plough and Teva; payment or honoraria from Aerocrine, Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, Regeneron Pharmaceuticals Inc., Sanofi and Teva; support for attending meetings from AstraZeneca, Chiesi, GSK, Regeneron Pharmaceuticals Inc., Sanofi, Teva and Napp Pharmaceuticals; and other financial or non‐financial interests from AstraZeneca, Boehringer Ingelheim, GSK, Regeneron Pharmaceuticals Inc., Sanofi and Teva. Maarten van den Berge received research grants from Genentech, GSK, Novartis, Roche and Sanofi. Rosa Faner received research grants from AstraZeneca, GSK, Instituto de Salud Carlos III—Spanish National Health Service and Menarini; consulting fees from GSK; and payment or honoraria from Chiesi.
Figures


Comment in
-
Adding some NOVELTY to treatable traits.Respirology. 2022 Nov;27(11):912-913. doi: 10.1111/resp.14341. Epub 2022 Aug 4. Respirology. 2022. PMID: 35926852 No abstract available.
References
-
- Halpin DMG, Criner GJ, Papi A, Singh D, Anzueto A, Martinez FJ, et al. Global initiative for the diagnosis, management, and prevention of chronic obstructive lung disease. the 2020 gold science committee report on covid‐19 and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;203(1):24–36. 10.1164/rccm.202009-3533SO. - DOI - PMC - PubMed
-
- Pavord ID, Beasley R, Agusti A, Anderson GP, Bel E, Brusselle G, et al. After asthma—redefining airways diseases. Lancet. 2017;391:350–400. - PubMed
-
- Agusti A, Bel E, Thomas M, Vogelmeier C, Brusselle G, Holgate ST, et al. Treatable traits: toward precision medicine of airway diseases. Eur Respir J. 2016;47:410–9. - PubMed
-
- Agustí A, Bafadhel M, Beasley R, Bel EH, Faner R, Gibson PG, et al. Precision medicine in airway diseases: moving to clinical practice. Eur Respir J. 2017;50:1–13. - PubMed

