Protomer Formation Can Aid the Structural Identification of Caffeine Metabolites
- PMID: 35861491
- PMCID: PMC9352149
- DOI: 10.1021/acs.analchem.2c00257
Protomer Formation Can Aid the Structural Identification of Caffeine Metabolites
Abstract
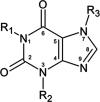
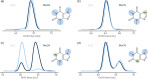
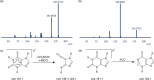
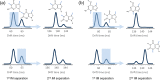
The structural annotation of isomeric metabolites remains a key challenge in untargeted electrospray ionization/high-resolution mass spectrometry (ESI/HRMS) metabolomic analysis. Many metabolites are polyfunctional compounds that may form protomers in electrospray ionization sources and therefore yield multiple peaks in ion mobility spectra. Protomer formation is strongly structure-specific. Here, we explore the possibility of using protomer formation for structural elucidation in metabolomics on the example of caffeine, its eight metabolites, and structurally related compounds. It is observed that two-thirds of the studied compounds formed high- and low-mobility species in high-resolution ion mobility. Structures in which proton hopping was hindered by a methyl group at the purine ring nitrogen (position 3) yielded structure-indicative fragments with collision-induced dissociation (CID) for high- and low-mobility ions. For compounds where such a methyl group was not present, a gas-phase equilibrium could be observed for tautomeric species with two-dimensional ion mobility. We show that the protomer formation and the gas-phase properties of the protomers can be related to the structure of caffeine metabolites and facilitate the identification of the structural isomers.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Collision-induced dissociation studies of caffeine in positive electrospray ionisation mass spectrometry using six deuterated isotopomers and one N1-ethylated homologue.Rapid Commun Mass Spectrom. 2013 Apr 30;27(8):885-95. doi: 10.1002/rcm.6520. Rapid Commun Mass Spectrom. 2013. PMID: 23495058
-
Solvent-Mediated Proton-Transfer Catalysis of the Gas-Phase Isomerization of Ciprofloxacin Protomers.J Am Soc Mass Spectrom. 2022 Feb 2;33(2):347-354. doi: 10.1021/jasms.1c00331. Epub 2022 Jan 11. J Am Soc Mass Spectrom. 2022. PMID: 35014802
-
Sodium adduct formation with graph-based machine learning can aid structural elucidation in non-targeted LC/ESI/HRMS.Anal Chim Acta. 2022 Apr 29;1204:339402. doi: 10.1016/j.aca.2021.339402. Epub 2021 Dec 27. Anal Chim Acta. 2022. PMID: 35397906
-
Decay mechanisms of protonated 4-quinolone antibiotics after electrospray ionization and ion activation.J Am Soc Mass Spectrom. 2014 Nov;25(11):1974-86. doi: 10.1007/s13361-014-0972-2. Epub 2014 Sep 9. J Am Soc Mass Spectrom. 2014. PMID: 25201456
-
Understanding of protomers/deprotomers by combining mass spectrometry and computation.Anal Bioanal Chem. 2023 Jul;415(18):3847-3862. doi: 10.1007/s00216-023-04574-1. Epub 2023 Feb 4. Anal Bioanal Chem. 2023. PMID: 36737499 Review.
Cited by
-
Impact of Protonation Sites on Collision-Induced Dissociation-MS/MS Using CIDMD Quantum Chemistry Modeling.J Chem Inf Model. 2024 Oct 14;64(19):7457-7469. doi: 10.1021/acs.jcim.4c00761. Epub 2024 Sep 27. J Chem Inf Model. 2024. PMID: 39329341
-
Traveling Wave Ion Mobility-Derived Collision Cross Section Database for Plant Specialized Metabolites: An Application to Ventilago harmandiana Pierre.J Proteome Res. 2022 Oct 7;21(10):2481-2492. doi: 10.1021/acs.jproteome.2c00413. Epub 2022 Sep 25. J Proteome Res. 2022. PMID: 36154058 Free PMC article.
-
Characterization of Anticancer Drug Protomers Using Electrospray Ionization and Ion Mobility Spectrometry-Mass Spectrometry.J Am Soc Mass Spectrom. 2024 Dec 4;35(12):2869-2876. doi: 10.1021/jasms.4c00233. Epub 2024 Sep 27. J Am Soc Mass Spectrom. 2024. PMID: 39355976 Free PMC article.
-
Recent advances in high-resolution traveling wave-based ion mobility separations coupled to mass spectrometry.Mass Spectrom Rev. 2025 Jul-Aug;44(4):581-598. doi: 10.1002/mas.21902. Epub 2024 Aug 1. Mass Spectrom Rev. 2025. PMID: 39087820 Free PMC article. Review.
-
Critical review on in silico methods for structural annotation of chemicals detected with LC/HRMS non-targeted screening.Anal Bioanal Chem. 2025 Jan;417(3):473-493. doi: 10.1007/s00216-024-05471-x. Epub 2024 Aug 14. Anal Bioanal Chem. 2025. PMID: 39138659 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

