Diagnostic Accuracy of the Truenat MTB Plus Assay and Comparison with the Xpert MTB/RIF Assay to Detect Tuberculosis among Hospital Outpatients in Cameroon
- PMID: 35861529
- PMCID: PMC9383115
- DOI: 10.1128/jcm.00155-22
Diagnostic Accuracy of the Truenat MTB Plus Assay and Comparison with the Xpert MTB/RIF Assay to Detect Tuberculosis among Hospital Outpatients in Cameroon
Abstract
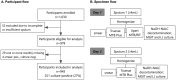
The Truenat MTB Plus assay is a rapid molecular test that has been recommended by the World Health Organization since 2020 as an initial test to detect tuberculosis (TB). The WHO highlighted the need to further evaluate assay performance to inform future recommendations, including in people living with HIV and compared to the Xpert MTB/RIF assay. We conducted a prospective evaluation of the diagnostic accuracy of the Truenat assay in Cameroon, a country with a high burden of HIV/TB. Adult outpatients were recruited at four hospitals; demographic information and medical history were collected, and participants produced two sputum specimens. Truenat and Xpert testing was performed on the same specimen, and performance was compared to TB culture as the reference standard. From November 2019 to December 2020, 945 participants were enrolled and included in the analysis. Among 251 participants with culture-positive TB, the sensitivity of Truenat MTB Plus was 91% (95% confidence interval [CI], 86 to 94%), similar to Xpert (90%; 95% CI, 86 to 93%). Among 74 HIV-positive participants with culture-positive TB, the sensitivity of Truenat MTB Plus was 85% (95% CI, 75 to 92%) compared to 81% for Xpert (95% CI, 70 to 89%). Among 47 participants with smear-negative TB, the sensitivity of Truenat MTB Plus was 55% (95% CI, 40 to 70%), similar to Xpert (53%; 95% CI, 38 to 68%). The specificity of Truenat MTB Plus was 96% (95% CI, 94 to 97%) compared to 99% (95% CI, 97 to 99%) for Xpert. For TB detection compared to the reference standard of TB culture, the performance of the Truenat MTB Plus assay was similar to that of Xpert in this population, including among people living with HIV.
Keywords: Mycobacterium tuberculosis; diagnostics; molecular methods.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- World Health Organization. 2020. Global tuberculosis report 2021. World Health Organization, Geneva, Switzerland. https://www.who.int/publications/i/item/9789240037021. Accessed 31 January 2022.
-
- World Health Organization. 2019. Global tuberculosis report 2020. World Health Organization, Geneva, Switzerland. https://www.who.int/publications/i/item/9789240013131. Accessed 31 January 2022.
-
- Reid MJA, Arinaminpathy N, Bloom A, Bloom BR, Boehme C, Chaisson R, Chin DP, Churchyard G, Cox H, Ditiu L, Dybul M, Farrar J, Fauci AS, Fekadu E, Fujiwara PI, Hallett TB, Hanson CL, Harrington M, Herbert N, Hopewell PC, Ikeda C, Jamison DT, Khan AJ, Koek I, Krishnan N, Motsoaledi A, Pai M, Raviglione MC, Sharman A, Small PM, Swaminathan S, Temesgen Z, Vassall A, Venkatesan N, van Weezenbeek K, Yamey G, Agins BD, Alexandru S, Andrews JR, Beyeler N, Bivol S, Brigden G, Cattamanchi A, Cazabon D, Crudu V, Daftary A, Dewan P, Doepel LK, Eisinger RW, Fan V, et al.. 2019. Building a tuberculosis-free world: the Lancet commission on tuberculosis. Lancet 393:1331–1384. doi:10.1016/S0140-6736(19)30024-8. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

