Cutaneous adverse reactions following COVID-19 vaccinations: A systematic review and meta-analysis
- PMID: 35861631
- PMCID: PMC9350270
- DOI: 10.1111/jocd.15261
Cutaneous adverse reactions following COVID-19 vaccinations: A systematic review and meta-analysis
Abstract
Background: COVID-19 vaccines are currently the most effective interventions in controlling and preventing severe disease progression. Dermatologic reactions to COVID-19 vaccinations may be rare among clinical trial participants. However, since global mass vaccination became a reality, these adverse effects may become more widespread, and different skin reactions would arise.
Objective: To systematically review the cutaneous adverse reactions in cases subject to vaccines for COVID-19.
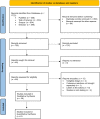
Methods: We searched the PubMed, SCOPUS, Web of Science, and Embase databases, identifying the relevant records and including the eligible observational ones. After assessing the methodological quality of the included studies, we qualitatively and quantitatively synthesized the data regarding the cutaneous side effects experienced by those in the studies' population.
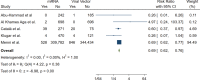
Results: Overall, 36 studies were included in our systematic review, with the majority being cross-sectional. We found that pain, erythema, and swelling were the most common local side effects, while different types of rashes, urticaria, and angioedema were the most non-local. Few cases also reported experiencing flare-ups of their underlying diseases or developing newly-onset diseases of various etiologies. Our meta-analyses also found that while viral vector-based vaccines are, though insignificantly, safer in injection site complaints, individuals who received mRNA vaccines developed significantly fewer non-local cutaneous adverse events.
Discussion: Cutaneous reactions to the COVID-19 vaccines are similar to common cutaneous drug eruptions and COVID-19 cutaneous manifestations. However, we believe that further high-quality research is needed to assess better how and why cutaneous reactions occur in different vaccines.
Keywords: COVID-19; cutaneous; skin; vaccination.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that no conflict of or competing interests existed or occurred in the conduction of this manuscript.
Figures



Comment in
-
Comment on "Cutaneous adverse reactions of COVID-19 vaccines: A systematic review".J Cosmet Dermatol. 2023 Mar;22(3):732-733. doi: 10.1111/jocd.15612. Epub 2023 Jan 27. J Cosmet Dermatol. 2023. PMID: 36705541 No abstract available.
References
-
- Coronavirus disease (COVID‐19): vaccines. https://www.who.int/news‐room/questions‐and‐answers/item/coronavirus‐dis...
-
- COVID‐19 vaccine tracker and landscape. https://www.who.int/publications/m/item/draft‐landscape‐of‐covid‐19‐cand...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

