Pentoxifylline as a therapeutic option for pre-eclampsia: a study on its placental effects
- PMID: 35861684
- PMCID: PMC9804511
- DOI: 10.1111/bph.15931
Pentoxifylline as a therapeutic option for pre-eclampsia: a study on its placental effects
Abstract
Background and purpose: Recently pentoxifylline, a non-selective phosphodiesterase inhibitor and adenosine receptor antagonist, has attracted much interest for the treatment of the increased vascular resistance and endothelial dysfunction in pre-eclampsia. We therefore investigated the placental transfer, vascular effects and anti-inflammatory actions of pentoxifylline in healthy and pre-eclamptic human placentas.
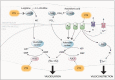
Experimental approach: The placental transfer and metabolism of pentoxifylline were studied using ex vivo placenta perfusion experiments. In wire myography experiments with chorionic plate arteries, pentoxifyllines vasodilator properties were investigated, focusing on the cGMP and cAMP pathways and adenosine receptors. Its effects on inflammatory factors were also studied in placental explants.
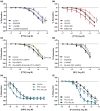
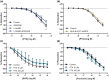
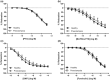
Key results: Pentoxifylline transferred from the maternal to foetal circulation, reaching identical concentrations. The placenta metabolized pentoxifylline into its active metabolite lisofylline (M1), which was released into both circulations. In healthy placentas, pentoxifylline potentiated cAMP- and cGMP-induced vasodilation, as well as causing vasodilation by adenosine A1 antagonism and via NO synthase and PKG. Pentoxifylline also reduced inflammatory factors secretion. In pre-eclamptic placentas, we observed that its vasodilator capacity was preserved, however not via NO-PKG but likely through adenosine signalling. Pentoxifylline neither potentiated vasodilation through cAMP and cGMP, nor suppressed the release of inflammatory factors from these placentas.
Conclusion and implications: Pentoxifylline is transferred across and metabolized by the placenta. Its beneficial effects on the NO pathway and inflammation are not retained in pre-eclampsia, limiting its application in this disease, although it could be useful for other placenta-related disorders. Future studies might focus on selective A1 receptor antagonists as a new treatment for pre-eclampsia.
Keywords: inflammation; nitric oxide; pentoxifylline; phosphodiesterase; placenta; pre-eclampsia; vasodilation.
© 2022 The Authors. British Journal of Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
We have no conflicts of interest to disclose.
Figures


References
-
- Alexander, S. P. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Abbracchio, M. P. , Alexander, W. , Al‐hosaini, K. , Bäck, M. , Barnes, N. M. , Bathgate, R. , … Ye, R. D. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: G protein‐coupled receptors. British Journal of Pharmacology, 178(S1), S27–S156. 10.1111/bph.15538 - DOI - PubMed
-
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Beuve, A. , Brouckaert, P. , Bryant, C. , Burnett, J. C. , Farndale, R. W. , Friebe, A. , Garthwaite, J. , … Waldman, S. A. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Catalytic receptors. British Journal of Pharmacology, 178(S1), S264–S312. 10.1111/bph.15541 - DOI - PubMed
-
- Alexander, S. P. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , Armstrong, J. F. , Faccenda, E. , Harding, S. D. , Pawson, A. J. , Southan, C. , Davies, J. A. , Boison, D. , Burns, K. E. , Dessauer, C. , Gertsch, J. , Helsby, N. A. , Izzo, A. A. , Koesling, D. , … Wong, S. S. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: Enzymes. British Journal of Pharmacology, 178(S1), S313–S411. 10.1111/bph.15542 - DOI - PubMed
-
- American College of Obstetricians and Gynecologists Task Force on Hypertension in Pregnancy . (2013). Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' task force on hypertension in pregnancy. Obstetrics and Gynecology, 122(5), 1122–1131. 10.1097/01.AOG.0000437382.03963.88 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

