The Combination of Mesyl-Phosphoramidate Inter-Nucleotide Linkages and 2'- O-Methyl in Selected Positions in the Antisense Oligonucleotide Enhances the Performance of RNaseH1 Active PS-ASOs
- PMID: 35861704
- PMCID: PMC9595634
- DOI: 10.1089/nat.2022.0005
The Combination of Mesyl-Phosphoramidate Inter-Nucleotide Linkages and 2'- O-Methyl in Selected Positions in the Antisense Oligonucleotide Enhances the Performance of RNaseH1 Active PS-ASOs
Abstract
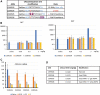
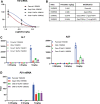
Antisense oligonucleotides (ASOs) that mediate RNA target degradation by RNase H1 are used as drugs to treat various diseases. Previously we found that introduction of a single 2'-O-methyl (2'-OMe) modification in position 2 of the central deoxynucleotide region of a gapmer phosphorothioate (PS) ASO, in which several residues at the termini are 2'-methoxyethyl, 2' constrained ethyl, or locked nucleic acid, dramatically reduced cytotoxicity with only modest effects on potency. More recently, we demonstrated that replacement of the PS linkage at position 2 or 3 in the gap with a mesyl-phosphoramidate (MsPA) linkage also significantly reduced toxicity without meaningful loss of potency and increased the elimination half-life of the ASOs. In this study, we evaluated the effects of the combination of MsPA linkages and 2'-OMe nucleotides on PS ASO performance. We found that two MsPA modifications at the 5' end of the gap or in the 3'-wing of a Gap 2'-OMe PS ASO substantially increased the activity of ASOs with OMe at position 2 of the gap without altering the safety profile. Such effects were observed with multiple sequences in cells and animals. Thus, the MsPA modification improves the RNase H1 cleavage rate of PS ASOs with a 2'-OMe in the gap, significantly reduces binding of proteins involved in cytotoxicity, and prolongs elimination half-lives.
Keywords: 2′-O-methyl; RNase H1; antisense oligonucleotide; mesyl-phosphoramidate.
Conflict of interest statement
All authors are employees of Ionis Pharmaceuticals.
Figures




Similar articles
-
Understanding the effect of controlling phosphorothioate chirality in the DNA gap on the potency and safety of gapmer antisense oligonucleotides.Nucleic Acids Res. 2020 Feb 28;48(4):1691-1700. doi: 10.1093/nar/gkaa031. Nucleic Acids Res. 2020. PMID: 31980820 Free PMC article.
-
Chemical modification of PS-ASO therapeutics reduces cellular protein-binding and improves the therapeutic index.Nat Biotechnol. 2019 Jun;37(6):640-650. doi: 10.1038/s41587-019-0106-2. Epub 2019 Apr 29. Nat Biotechnol. 2019. PMID: 31036929
-
Hsp90 protein interacts with phosphorothioate oligonucleotides containing hydrophobic 2'-modifications and enhances antisense activity.Nucleic Acids Res. 2016 May 5;44(8):3892-907. doi: 10.1093/nar/gkw144. Epub 2016 Mar 3. Nucleic Acids Res. 2016. PMID: 26945041 Free PMC article.
-
Cellular uptake and trafficking of antisense oligonucleotides.Nat Biotechnol. 2017 Mar;35(3):230-237. doi: 10.1038/nbt.3779. Epub 2017 Feb 27. Nat Biotechnol. 2017. PMID: 28244996 Review.
-
Small Drugs, Huge Impact: The Extraordinary Impact of Antisense Oligonucleotides in Research and Drug Development.Molecules. 2022 Jan 15;27(2):536. doi: 10.3390/molecules27020536. Molecules. 2022. PMID: 35056851 Free PMC article. Review.
Cited by
-
Towards Personalized Allele-Specific Antisense Oligonucleotide Therapies for Toxic Gain-of-Function Neurodegenerative Diseases.Pharmaceutics. 2022 Aug 16;14(8):1708. doi: 10.3390/pharmaceutics14081708. Pharmaceutics. 2022. PMID: 36015334 Free PMC article. Review.
-
Chemical Modifications in Nucleic Acid Therapeutics.Methods Mol Biol. 2025;2965:57-126. doi: 10.1007/978-1-0716-4742-4_3. Methods Mol Biol. 2025. PMID: 40877500 Review.
-
Antisense oligonucleotide is a promising intervention for liver diseases.Front Pharmacol. 2022 Dec 9;13:1061842. doi: 10.3389/fphar.2022.1061842. eCollection 2022. Front Pharmacol. 2022. PMID: 36569303 Free PMC article. Review.
-
Metabolic Stability and Targeted Delivery of Oligonucleotides: Advancing RNA Therapeutics Beyond The Liver.J Med Chem. 2025 Apr 10;68(7):6870-6896. doi: 10.1021/acs.jmedchem.4c02528. Epub 2025 Jan 8. J Med Chem. 2025. PMID: 39772535 Free PMC article. Review.
-
Endogenous Ribonucleases: Therapeutic Targeting of the Transcriptome Through Oligonucleotide-Triggered RNA Inactivation.Biomolecules. 2025 Jul 4;15(7):965. doi: 10.3390/biom15070965. Biomolecules. 2025. PMID: 40723836 Free PMC article. Review.
References
-
- Kakiuchi-Kiyota S, Whiteley LO, Ryan AM, and Mathialagan N. (2016). Development of a method for profiling protein interactions with LNA-modified antisense oligonucleotides using protein microarrays. Nucleic Acid Ther 26:93–101. - PubMed
-
- Shen W, De Hoyos CL, Migawa MT, Vickers TA, Sun H, Low A, Bell TA, 3rd, Rahdar M, Mukhopadhyay S, et al. (2019). Chemical modification of PS-ASO therapeutics reduces cellular protein-binding and improves the therapeutic index. Nat Biotechnol 37:640–650. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
