Flavin Containing Monooxygenase 2 Prevents Cardiac Fibrosis via CYP2J3-SMURF2 Axis
- PMID: 35861735
- PMCID: PMC9932658
- DOI: 10.1161/CIRCRESAHA.122.320538
Flavin Containing Monooxygenase 2 Prevents Cardiac Fibrosis via CYP2J3-SMURF2 Axis
Abstract
Background: Cardiac fibrosis is a common pathological feature associated with adverse clinical outcome in postinjury remodeling and has no effective therapy. Using an unbiased transcriptome analysis, we identified FMO2 (flavin-containing monooxygenase 2) as a top-ranked gene dynamically expressed following myocardial infarction (MI) in hearts across different species including rodents, nonhuman primates, and human. However, the functional role of FMO2 in cardiac remodeling is largely unknown.
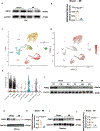
Methods: Single-nuclei transcriptome analysis was performed to identify FMO2 after MI; FMO2 ablation rats were generated both in genetic level using the CRISPR-cas9 (clustered regularly interspaced short palindromic repeats/clustered regularly interspaced short palindromic repeat-associated 9) technology and lentivirus-mediated manner. Gain-of-function experiments were conducted using postn-promoter FMO2, miR1a/miR133a-FMO2 lentivirus, and enzymatic activity mutant FMO2 lentivirus after MI.
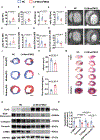
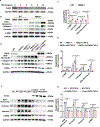
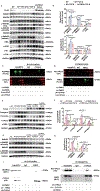
Results: A significant downregulation of FMO2 was consistently observed in hearts after MI in rodents, nonhuman primates, and patients. Single-nuclei transcriptome analysis showed cardiac expression of FMO2 was enriched in fibroblasts rather than myocytes. Elevated spontaneous tissue fibrosis was observed in the FMO2-null animals without external stress. In contrast, fibroblast-specific expression of FMO2 markedly reduced cardiac fibrosis following MI in rodents and nonhuman primates associated with diminished SMAD2/3 phosphorylation. Unexpectedly, the FMO2-mediated regulation in fibrosis and SMAD2/3 signaling was independent of its enzymatic activity. Rather, FMO2 was detected to interact with CYP2J3 (cytochrome p450 superfamily 2J3). Binding of FMO2 to CYP2J3 disrupted CYP2J3 interaction with SMURF2 (SMAD-specific E3 ubiquitin ligase 2) in cytosol, leading to increased cytoplasm to nuclear translocation of SMURF2 and consequent inhibition of SMAD2/3 signaling.
Conclusions: Loss of FMO2 is a conserved molecular signature in postinjury hearts. FMO2 possesses a previously uncharacterized enzyme-independent antifibrosis activity via the CYP2J3-SMURF2 axis. Restoring FMO2 expression exerts potent ameliorative effect against fibrotic remodeling in postinjury hearts from rodents to nonhuman primates. Therefore, FMO2 is a potential therapeutic target for treating cardiac fibrosis following injury.
Keywords: cytosol; downregulation; fibrosis; humans; myocardial infarction.
Figures



References
-
- Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–582. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

