Age-Independent Cardiac Protection by Pharmacological Activation of Beclin-1 During Endotoxemia and Its Association With Energy Metabolic Reprograming in Myocardium-A Targeted Metabolomics Study
- PMID: 35861821
- PMCID: PMC9707816
- DOI: 10.1161/JAHA.122.025310
Age-Independent Cardiac Protection by Pharmacological Activation of Beclin-1 During Endotoxemia and Its Association With Energy Metabolic Reprograming in Myocardium-A Targeted Metabolomics Study
Abstract
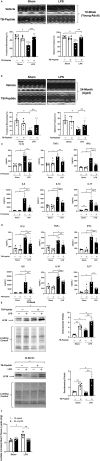
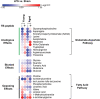
Background We showed that Beclin-1-dependent autophagy protects the heart in young and adult mice that underwent endotoxemia. Herein, we compared the potential therapeutic effects of Beclin-1 activating peptide, TB-peptide, on endotoxemia-induced cardiac outcomes in young adult and aged mice. We further evaluated lipopolysaccharide (lipopolysaccharide)-induced and TB-peptide treatment-mediated alterations in myocardial metabolism. Methods and Results C57BL/6J mice that were 10 weeks and 24 months old were challenged by lipopolysaccharide using doses at which cardiac dysfunction occurred. Following the treatment of TB-peptide or control vehicle, heart contractility, circulating cytokines, and myocardial autophagy were evaluated. We detected that TB-peptide boosted autophagy, attenuated cytokines, and improved cardiac performance in both young and aged mice during endotoxemia. A targeted metabolomics assay was designed to detect a pool of 361 known metabolites, of which 156 were detected in at least 1 of the heart tissue samples. Lipopolysaccharide-induced impairments were found in glucose and amino acid metabolisms in mice of all ages, and TB-peptide ameliorated these alterations. However, lipid metabolites were upregulated in the young group but moderately downregulated in the aged by lipopolysaccharide, suggesting an age-dependent response. TB-peptide mitigated lipopolysaccharide-mediated trend of lipids in the young mice but had little effect on the aged. (Study registration: Project DOI: https://doi.org/10.21228/M8K11W). Conclusions Pharmacological activation of Beclin-1 by TB-peptide is cardiac protective in both young and aged population during endotoxemia, suggest a therapeutic potential for sepsis-induced cardiomyopathy. Metabolomics analysis suggests that an age-independent protection by TB-peptide is associated with reprograming of energy production via glucose and amino acid metabolisms.
Keywords: Beclin‐1; autophagy; cardiac function; cardiac metabolism; endotoxemia; sepsis.
Figures


Similar articles
-
Beclin-1-Dependent Autophagy Protects the Heart During Sepsis.Circulation. 2018 Nov 13;138(20):2247-2262. doi: 10.1161/CIRCULATIONAHA.117.032821. Circulation. 2018. PMID: 29853517 Free PMC article.
-
Beclin-1-Dependent Autophagy Improves Outcomes of Pneumonia-Induced Sepsis.Front Cell Infect Microbiol. 2021 Jun 15;11:706637. doi: 10.3389/fcimb.2021.706637. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34211859 Free PMC article.
-
Beclin-1 improves mitochondria-associated membranes in the heart during endotoxemia.FASEB Bioadv. 2021 Jan 25;3(3):123-135. doi: 10.1096/fba.2020-00039. eCollection 2021 Mar. FASEB Bioadv. 2021. PMID: 33733054 Free PMC article.
-
The endotoxemia cardiac dysfunction is attenuated by AMPK/mTOR signaling pathway regulating autophagy.Biochem Biophys Res Commun. 2017 Oct 21;492(3):520-527. doi: 10.1016/j.bbrc.2017.08.034. Epub 2017 Aug 12. Biochem Biophys Res Commun. 2017. PMID: 28807827 Free PMC article.
-
Cardiac dysfunction, mitochondrial architecture, energy production, and inflammatory pathways: Interrelated aspects in endotoxemia and sepsis.Int J Biochem Cell Biol. 2016 Dec;81(Pt B):307-314. doi: 10.1016/j.biocel.2016.07.032. Epub 2016 Jul 28. Int J Biochem Cell Biol. 2016. PMID: 27477311 Review.
Cited by
-
Myocardial pyruvate dehydrogenase kinase 4 drives sex-specific cardiac responses to endotoxemia.JCI Insight. 2025 Jul 8;10(13):e191649. doi: 10.1172/jci.insight.191649. eCollection 2025 Jul 8. JCI Insight. 2025. PMID: 40626362 Free PMC article.
-
Beclin-1 dependent autophagy improves renal outcomes following Unilateral Ureteral Obstruction (UUO) injury.Front Immunol. 2023 Feb 16;14:1104652. doi: 10.3389/fimmu.2023.1104652. eCollection 2023. Front Immunol. 2023. PMID: 36875088 Free PMC article.
-
Age-Related Mitochondrial Alterations Contribute to Myocardial Responses During Sepsis.Cells. 2025 Aug 7;14(15):1221. doi: 10.3390/cells14151221. Cells. 2025. PMID: 40801652 Free PMC article.
-
Utilizing omics technologies in the investigation of sepsis-induced cardiomyopathy.Int J Cardiol Heart Vasc. 2024 Jul 30;54:101477. doi: 10.1016/j.ijcha.2024.101477. eCollection 2024 Oct. Int J Cardiol Heart Vasc. 2024. PMID: 39171080 Free PMC article. Review.
-
Multi-Omics and Network-Based Drug Repurposing for Septic Cardiomyopathy.Pharmaceuticals (Basel). 2025 Jan 2;18(1):43. doi: 10.3390/ph18010043. Pharmaceuticals (Basel). 2025. PMID: 39861106 Free PMC article.
References
-
- Levy MM, Dellinger RP, Townsend SR, Linde‐Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay G, Beale R, et al. The Surviving Sepsis Campaign: results of an international guideline‐based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38:367–374. doi: 10.1097/CCM.0b013e3181cb0cdc - DOI - PubMed
-
- Drosatos K, Drosatos‐Tampakaki Z, Khan R, Homma S, Schulze PC, Zannis VI, Goldberg IJ. Inhibition of c‐Jun‐N‐terminal kinase increases cardiac peroxisome proliferator‐activated receptor alpha expression and fatty acid oxidation and prevents lipopolysaccharide‐induced heart dysfunction. J Biol Chem. 2011;286:36331–36339. doi: 10.1074/jbc.M111.272146 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

