Candidate Plasma Biomarkers to Detect Anthracycline-Related Cardiomyopathy in Childhood Cancer Survivors: A Case Control Study in the Dutch Childhood Cancer Survivor Study
- PMID: 35861824
- PMCID: PMC9707839
- DOI: 10.1161/JAHA.121.025935
Candidate Plasma Biomarkers to Detect Anthracycline-Related Cardiomyopathy in Childhood Cancer Survivors: A Case Control Study in the Dutch Childhood Cancer Survivor Study
Abstract
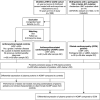
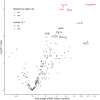
Background Plasma biomarkers may aid in the detection of anthracycline-related cardiomyopathy (ACMP). However, the currently available biomarkers have limited diagnostic value in long-term childhood cancer survivors. This study sought to identify diagnostic plasma biomarkers for ACMP in childhood cancer survivors. Methods and Results We measured 275 plasma proteins in 28 ACMP cases with left ventricular ejection fraction <45%, 29 anthracycline-treated controls with left ventricular ejection fraction ≥53% matched on sex, time after cancer, and anthracycline dose, and 29 patients with genetically determined dilated cardiomyopathy with left ventricular ejection fraction <45%. Multivariable linear regression was used to identify differentially expressed proteins. Elastic net model, including clinical characteristics, was used to assess discrimination of proteins diagnostic for ACMP. NT-proBNP (N-terminal pro-B-type natriuretic peptide) and the inflammatory markers CCL19 (C-C motif chemokine ligands 19) and CCL20, PSPD (pulmonary surfactant protein-D), and PTN (pleiotrophin) were significantly upregulated in ACMP compared with controls. An elastic net model selected 45 proteins, including NT-proBNP, CCL19, CCL20 and PSPD, but not PTN, that discriminated ACMP cases from controls with an area under the receiver operating characteristic curve (AUC) of 0.78. This model was not superior to a model including NT-proBNP and clinical characteristics (AUC=0.75; P=0.766). However, when excluding 8 ACMP cases with heart failure, the full model was superior to that including only NT-proBNP and clinical characteristics (AUC=0.75 versus AUC=0.50; P=0.022). The same 45 proteins also showed good discrimination between dilated cardiomyopathy and controls (AUC=0.89), underscoring their association with cardiomyopathy. Conclusions We identified 3 specific inflammatory proteins as candidate plasma biomarkers for ACMP in long-term childhood cancer survivors and demonstrated protein overlap with dilated cardiomyopathy.
Keywords: anthracycline‐related cardiomyopathy; biomarkers; cancer therapy–related cardiac dysfunction; cardio‐oncology; chemokine ligands; childhood cancer survivors.
Figures
References
-
- Feijen E, Font‐Gonzalez A, Van der Pal HJH, Kok WEM, Geskus RB, Ronckers CM, Bresters D, van Dalen EC, van Dulmen‐den BE, van den Berg MH, et al. Risk and temporal changes of heart failure among 5‐year childhood cancer survivors: a DCOG‐LATER study. J Am Heart Assoc. 2019;8:e009122. doi: 10.1161/jaha.118.009122 - DOI - PMC - PubMed
-
- Armenian SH, Hudson MM, Mulder RL, Chen MH, Constine LS, Dwyer M, Nathan PC, Tissing WJ, Shankar S, Sieswerda E, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the international late effects of childhood cancer guideline harmonization group. Lancet Oncol. 2015;16:e123–e136. doi: 10.1016/s1470-2045(14)70409-7 - DOI - PMC - PubMed
-
- Leerink JM, Verkleij SJ, Feijen EAM, Mavinkurve‐Groothuis AMC, Pourier MS, Ylänen K, Tissing WJE, Louwerens M, van den Heuvel MM, van Dulmen‐den BE, et al. Biomarkers to diagnose ventricular dysfunction in childhood cancer survivors: a systematic review. Heart (British Cardiac Society). 2019;105:210–216. doi: 10.1136/heartjnl-2018-313634 - DOI - PubMed
-
- Toro‐Salazar OH, Lee JH, Zellars KN, Perreault PE, Mason KC, Wang Z, Hor KN, Gillan E, Zeiss CJ, Gatti DM, et al. Use of integrated imaging and serum biomarker profiles to identify subclinical dysfunction in pediatric cancer patients treated with anthracyclines. Cardio‐Oncol. 2018;4:4. doi: 10.1186/s40959-018-0030-5 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

