Water Reduction and Dihydrogen Addition in Aqueous Conditions With ansa-Phosphinoborane
- PMID: 35861909
- PMCID: PMC9804508
- DOI: 10.1002/chem.202201927
Water Reduction and Dihydrogen Addition in Aqueous Conditions With ansa-Phosphinoborane
Abstract
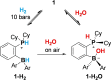
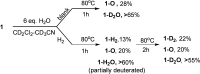
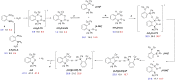
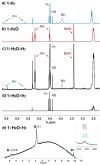
Ortho-phenylene-bridged phosphinoborane (2,6-Cl2 Ph)2 B-C6 H4 -PCy2 1 was synthesized in three steps from commercially available starting materials. 1 reacts with H2 or H2 O under mild conditions to form corresponding zwitterionic phosphonium borates 1-H2 or 1-H2 O. NMR studies revealed both reactions to be remarkably reversible. Thus, when exposed to H2 , 1-H2 O partially converts to 1-H2 even in the presence of multiple equivalents of water in the solution. The addition of parahydrogen to 1 leads to nuclear spin hyperpolarization both in dry and hydrous solvents, confirming the dissociation of 1-H2 O to free 1. These observations were supported by computational studies indicating that the formation of 1-H2 and 1-H2 O from 1 are thermodynamically favored. Unexpectedly, 1-H2 O can release molecular hydrogen to form phosphine oxide 1-O. Kinetic, mechanistic, and computational (DFT) studies were used to elucidate the unique "umpolung" water reduction mechanism.
Keywords: DFT; frustrated Lewis pairs; hydrogen activation; mechanism; umpolung of proton; water reduction.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

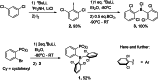






References
-
- None
-
- Stephan D. W., Erker G., Chem. Sci. 2014, 5, 2625–2641;
-
- Stephan D. W., Science 2016, 354, aaf7229;
-
- Melen R. L., Angew. Chem. Int. Ed. 2018, 57, 880–882; - PubMed
- Angew. Chem. 2018, 130, 890–892;
-
- Jupp A. R., Stephan D. W., Trends Chem. 2019, 1, 35–48.
Grants and funding
LinkOut - more resources
Full Text Sources

